CIKS/DDX3X interaction controls the stability of the Zc3h12a mRNA induced by IL-17
- PMID: 25710910
- PMCID: PMC4369453
- DOI: 10.4049/jimmunol.1401589
CIKS/DDX3X interaction controls the stability of the Zc3h12a mRNA induced by IL-17
Abstract
IL-17 is a proinflammatory cytokine that promotes the expression of different cytokines and chemokines via the induction of gene transcription and the posttranscriptional stabilization of mRNAs. In this study, we show that IL-17 increases the half-life of the Zc3h12a mRNA via interaction of the adaptor protein CIKS with the DEAD box protein DDX3X. IL-17 stimulation promotes the formation of a complex between CIKS and DDX3X, and this interaction requires the helicase domain of DDX3X but not its ATPase activity. DDX3X knockdown decreases the IL-17-induced stability of Zc3h12a without affecting the stability of other mRNAs. IKKε, TNFR-associated factor 2, and TNFR-associated factor 5 were also required to mediate the IL-17-induced Zc3h12a stabilization. DDX3X directly binds the Zc3h12a mRNA after IL-17 stimulation. Collectively, our findings define a novel, IL-17-dependent mechanism regulating the stabilization of a selected mRNA.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures


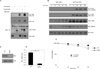


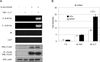
Similar articles
-
Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF).Nat Immunol. 2011 Aug 7;12(9):853-60. doi: 10.1038/ni.2081. Nat Immunol. 2011. PMID: 21822258 Free PMC article.
-
CIKS/Act1-mediated signaling by IL-17 cytokines in context: implications for how a CIKS gene variant may predispose to psoriasis.J Immunol. 2012 Jun 15;188(12):5906-14. doi: 10.4049/jimmunol.1103233. Epub 2012 May 11. J Immunol. 2012. PMID: 22581863 Free PMC article.
-
The perplexities of the ZC3H12A self-mRNA regulation.Acta Biochim Pol. 2016;63(3):411-5. doi: 10.18388/abp.2016_1325. Epub 2016 Aug 5. Acta Biochim Pol. 2016. PMID: 27494113
-
The activation and regulation of IL-17 receptor mediated signaling.Cytokine. 2013 May;62(2):175-82. doi: 10.1016/j.cyto.2013.03.014. Epub 2013 Apr 1. Cytokine. 2013. PMID: 23557798 Review.
-
mRNA degradation by the endoribonuclease Regnase-1/ZC3H12a/MCPIP-1.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):708-13. doi: 10.1016/j.bbagrm.2013.03.001. Epub 2013 Mar 13. Biochim Biophys Acta. 2013. PMID: 23500036 Review.
Cited by
-
Terminal uridyltransferase 7 regulates TLR4-triggered inflammation by controlling Regnase-1 mRNA uridylation and degradation.Nat Commun. 2021 Jun 29;12(1):3878. doi: 10.1038/s41467-021-24177-7. Nat Commun. 2021. PMID: 34188032 Free PMC article.
-
Genetic variants associated mRNA stability in lung.BMC Genomics. 2022 Mar 11;23(1):196. doi: 10.1186/s12864-022-08405-y. BMC Genomics. 2022. PMID: 35272635 Free PMC article.
-
IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a.Sci Signal. 2018 Oct 9;11(551):eaat4617. doi: 10.1126/scisignal.aat4617. Sci Signal. 2018. PMID: 30301788 Free PMC article.
-
Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications.Cold Spring Harb Perspect Biol. 2018 Apr 2;10(4):a028522. doi: 10.1101/cshperspect.a028522. Cold Spring Harb Perspect Biol. 2018. PMID: 28620097 Free PMC article. Review.
-
RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins.Nat Rev Immunol. 2017 Feb;17(2):130-143. doi: 10.1038/nri.2016.129. Epub 2016 Dec 19. Nat Rev Immunol. 2017. PMID: 27990022 Free PMC article. Review.
References
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 2000;164:2832–2838. - PubMed
-
- Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Bérard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10(7):778–785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

