Versatile communication strategies among tandem WW domain repeats
- PMID: 25710931
- PMCID: PMC4436281
- DOI: 10.1177/1535370214566558
Versatile communication strategies among tandem WW domain repeats
Abstract
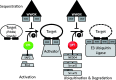
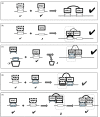
Interactions mediated by short linear motifs in proteins play major roles in regulation of cellular homeostasis since their transient nature allows for easy modulation. We are still far from a full understanding and appreciation of the complex regulation patterns that can be, and are, achieved by this type of interaction. The fact that many linear-motif-binding domains occur in tandem repeats in proteins indicates that their mutual communication is used extensively to obtain complex integration of information toward regulatory decisions. This review is an attempt to overview, and classify, different ways by which two and more tandem repeats cooperate in binding to their targets, in the well-characterized family of WW domains and their corresponding polyproline ligands.
Keywords: WW domain; WW domain containing oxidoreductase; Yes-associated protein; cooperative binding; peptide-mediated interactions; protein domain repeats; regulation of signaling.
© 2015 by the Society for Experimental Biology and Medicine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

