CD160 is essential for NK-mediated IFN-γ production
- PMID: 25711213
- PMCID: PMC4354368
- DOI: 10.1084/jem.20131601
CD160 is essential for NK-mediated IFN-γ production
Abstract
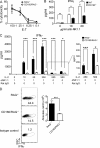
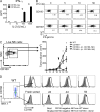
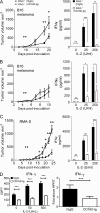
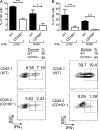
NK-derived cytokines play important roles for natural killer (NK) function, but how the cytokines are regulated is poorly understood. CD160 is expressed on activated NK or T cells in humans but its function is unknown. We generated CD160-deficient mice to probe its function. Although CD160(-/-) mice showed no abnormalities in lymphocyte development, the control of NK-sensitive tumors was severely compromised in CD160(-/-) mice. Surprisingly, the cytotoxicity of NK cells was not impaired, but interferon-γ (IFN-γ) secretion by NK cells was markedly reduced in CD160(-/-) mice. Functionally targeting CD160 signaling with a soluble CD160-Ig also impaired tumor control and IFN-γ production, suggesting an active role of CD160 signaling. Using reciprocal bone marrow transfer and cell culture, we have identified the intrinsic role of CD160 on NK cells, as well as its receptor on non-NK cells, for regulating cytokine production. To demonstrate sufficiency of the CD160(+) NK cell subset in controlling NK-dependent tumor growth, intratumoral transfer of the CD160(+) NK fraction led to tumor regression in CD160(-/-) tumor-bearing mice, indicating demonstrable therapeutic potential for controlling early tumors. Therefore, CD160 is not only an important biomarker but also functionally controls cytokine production by NK cells.
© 2015 Tu et al.
Figures



References
-
- Agrawal S., Marquet J., Freeman G.J., Tawab A., Bouteiller P.L., Roth P., Bolton W., Ogg G., Boumsell L., and Bensussan A.. 1999. Cutting edge: MHC class I triggering by a novel cell surface ligand costimulates proliferation of activated human T cells. J. Immunol. 162:1223–1226. - PubMed
-
- Altman J.D., and Davis M.M.. 2003. MHC-peptide tetramers to visualize antigen-specific T cells. Curr. Protoc. Immunol. Chapter 17:3. - PubMed
-
- Barakonyi A., Rabot M., Marie-Cardine A., Aguerre-Girr M., Polgar B., Schiavon V., Bensussan A., and Le Bouteiller P.. 2004. Cutting edge: engagement of CD160 by its HLA-C physiological ligand triggers a unique cytokine profile secretion in the cytotoxic peripheral blood NK cell subset. J. Immunol. 173:5349–5354 10.4049/jimmunol.173.9.5349 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

