Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling
- PMID: 25712125
- PMCID: PMC4401644
- DOI: 10.1158/0008-5472.CAN-14-3621
Hyperthermia Sensitizes Glioma Stem-like Cells to Radiation by Inhibiting AKT Signaling
Abstract
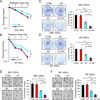
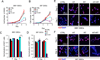
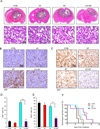
Glioma stem-like cells (GSC) are a subpopulation of cells in tumors that are believed to mediate self-renewal and relapse in glioblastoma (GBM), the most deadly form of primary brain cancer. In radiation oncology, hyperthermia is known to radiosensitize cells, and it is reemerging as a treatment option for patients with GBM. In this study, we investigated the mechanisms of hyperthermic radiosensitization in GSCs by a phospho-kinase array that revealed the survival kinase AKT as a critical sensitization determinant. GSCs treated with radiation alone exhibited increased AKT activation, but the addition of hyperthermia before radiotherapy reduced AKT activation and impaired GSC proliferation. Introduction of constitutively active AKT in GSCs compromised hyperthermic radiosensitization. Pharmacologic inhibition of PI3K further enhanced the radiosensitizing effects of hyperthermia. In a preclinical orthotopic transplant model of human GBM, thermoradiotherapy reduced pS6 levels, delayed tumor growth, and extended animal survival. Together, our results offer a preclinical proof-of-concept for further evaluation of combined hyperthermia and radiation for GBM treatment.
©2015 American Association for Cancer Research.
Conflict of interest statement
Authors report no conflict of interest.
Figures


References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet oncology. 2009;10(5):459–466. - PubMed
-
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. International journal of radiation oncology, biology, physics. 1979;5(10):1725–1731. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

