Programming and reprogramming a human heart cell
- PMID: 25712211
- PMCID: PMC4369310
- DOI: 10.15252/embj.201490563
Programming and reprogramming a human heart cell
Abstract
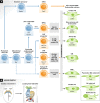
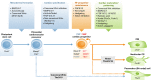
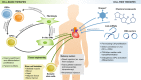
The latest discoveries and advanced knowledge in the fields of stem cell biology and developmental cardiology hold great promise for cardiac regenerative medicine, enabling researchers to design novel therapeutic tools and approaches to regenerate cardiac muscle for diseased hearts. However, progress in this arena has been hampered by a lack of reproducible and convincing evidence, which at best has yielded modest outcomes and is still far from clinical practice. To address current controversies and move cardiac regenerative therapeutics forward, it is crucial to gain a deeper understanding of the key cellular and molecular programs involved in human cardiogenesis and cardiac regeneration. In this review, we consider the fundamental principles that govern the "programming" and "reprogramming" of a human heart cell and discuss updated therapeutic strategies to regenerate a damaged heart.
Keywords: cardiac progenitor cell; cardiac regeneration; cardiomyocyte proliferation; embryonic heart field; reprogramming.
© 2015 The Authors.
Figures

References
-
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. - PubMed
-
- Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

