Cyclin O (Ccno) functions during deuterosome-mediated centriole amplification of multiciliated cells
- PMID: 25712475
- PMCID: PMC4406653
- DOI: 10.15252/embj.201490805
Cyclin O (Ccno) functions during deuterosome-mediated centriole amplification of multiciliated cells
Abstract
Mucociliary clearance and fluid transport along epithelial surfaces are carried out by multiciliated cells (MCCs). Recently, human mutations in Cyclin O (CCNO) were linked to severe airway disease. Here, we show that Ccno expression is restricted to MCCs and the genetic deletion of Ccno in mouse leads to reduced numbers of multiple motile cilia and characteristic phenotypes of MCC dysfunction including severe hydrocephalus and mucociliary clearance deficits. Reduced cilia numbers are caused by compromised generation of centrioles at deuterosomes, which serve as major amplification platform for centrioles in MCCs. Ccno-deficient MCCs fail to sufficiently generate deuterosomes, and only reduced numbers of fully functional centrioles that undergo maturation to ciliary basal bodies are formed. Collectively, this study implicates CCNO as first known regulator of deuterosome formation and function for the amplification of centrioles in MCCs.
Keywords: Ccno; centriole amplification; deuterosomes; mouse; multiciliated cells.
© 2015 The Authors.
Figures
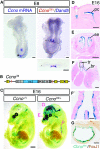
A Ccno mRNA is expressed in the embryonic node at E8 as shown by in situ hybridization. Occasionally, additional expression is observed at the posterior tip of the embryo (asterisk). Double labelling by staining for LacZ expression from the CcnoTA allele (shown in B) and in situ hybridization for Dand5, marking crown cells at the circumference of the node. Ccno expression is restricted to ciliated, ventral pit cells of the node as detailed in the transverse section at the indicated plane.
B Schematic of the CcnoTA targeted LacZ knock-in allele used in (A) and (C–G).
C Whole embryo X-Gal staining of E16 CcnoTA/+ and wild-type control embryos and indicated section planes of (D–F').
D–F Histological sections of LacZ-stained CcnoTA/+ embryo at E16 reveal Ccno expression in the epithelium of (D) plexus choroideus and ependyme, (E) snout epithelium, and (F, F') trachea and bronchi. pc, plexus choroideus; se, snout epithelium; tr, trachea; and br, bronchus.
G Co-expression of Ccno and Foxj1 in multiciliated cells of the trachea shown by double staining for X-Gal and immunohistochemistry using a FOXJ1-specific antibody.

A Ccno-deficient mice develop severe hydrocephalus resulting in characteristic head deformation at P21 (arrow).
B,C Cranial MRI analysis (B) shows the drastically enlarged ventricular cavity (marked by V) and diminished cerebral cortex tissue, (C) also seen when brains are cut in coronal orientation.
D,D' Alcian-blue staining of paranasal cavities reveals mucus congestion along the nasal epithelium (arrows).
E Survival table of different genotypes at embryonic stages (E13–E17) and at P21 from offspring of heterozygous CcnoRA/+ intercrosses. 11% of CcnoRA/RA homozygous animals die between E17 and P21. Of CcnoRA/RA homozygous animals at P21, roughly 60% develop severe hydrocephalus (20/35 animals), and 40% (14/35) appear grossly unaffected.
F,F' Scanning electron microscopy (SEM) of P21 adult trachea shows reduced numbers of cilia of Ccno-deficient MCCs. Remaining cilia are found in the central regions of the cell surface, and cell margins frequently lack the ciliary decoration (arrows).
G Transmission electron microscopy (TEM) of wild-type and CcnoRA/RA MCCs from adult trachea. Ccno-deficient MCCs show reduced numbers of basal bodies and cilia that correctly docked to the apical cell surface (arrows). Ectopic electron-dense material is found within the cytoplasm of Ccno-deficient MCCs (arrowheads).

A–H X-Gal-staining of (A–C) control and (D–H) CcnoTA/+ embryonic lungs at indicated stages showing onset of Ccno expression from E13 in the proximal trachea and in the main bronchi. Expression is extending to more distal regions, and from E16 staining is also found in bronchioli.
I X-Gal-staining indicating LacZ expression from the CcnoTA/+ allele in mTEC cultures after 5 days of differentiation-onset by switching to air–liquid interface (ALI) conditions (ALI d5).
J Transcript levels of Ccno and indicated genes with known functions for the generation of multiple cilia during mTEC differentiation. mRNA levels from three independent experiments were measured by qRT–PCR and levels of expression set as 1 on day 0 of ALI cultures. Scales for Ccno and Deup1 are indicated on the left, and for Cep152, Cep63 and Ift57 on the right side.
K Relative mRNA expression levels for indicated genes were measured by qRT–PCR in wild-type and Ccno-deficient mTEC cultures at indicated days after differentiation-onset. Relative values were calculated as in (J) relative to day 0 of ALI cultures.
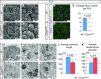
A–B' SEM of tracheae from (A, A') wild-type and (B, B') CcnoRA/RA embryos at E17. In comparison to wild-type MCCs, Ccno-deficient MCCs almost completely lack formation of multiple cilia and only single, or doublet (arrow) cilia are observed at E17.
C–E The lack of multiple cilia at E17 stages is reflected by the almost complete absence of acetylated α-tubulin staining as marker for axonemes in (D) Ccno-deficient MCCs of E17 embryonic trachea as quantified in (E). FOV, field of view.
F–I TEM of MCCs from E17 embryonic tracheae shows deuterosomes with forming procentrioles in (F, F', H) Ccno-proficient and (G, G', I) Ccno-deficient MCCs. Asterisks in (F, G) indicate microvilli that extend from the apical cell surface. (F', G') Deuterosomes in Ccno-deficient MCCs are significantly enlarged (identical size bars in F' and G') and show irregular morphology, which is different to the annular shape of wild-type deuterosomes as seen in (F', H). Procentrioles (arrowheads in F' and I) that are found at deuterosomes of (I) Ccno-deficient MCCs were recurrently found being shorter and appeared less structured in comparison to (F', H) wild-type cells.
J The average length of procentrioles was quantified for wild-type and Ccno-deficient MCCs of E17 tracheae (49 procentrioles in n = 3 independent samples for wild-types and 47 procentrioles in n = 3 independent samples for Ccno-deficient MCCs).
K The diameter of deuterosomes (41 deuterosomes in n = 3 independent samples for wild-types; 38 deuterosomes in n = 5 independent samples for Ccno-deficient MCCs) was measured in wild-type and Ccno-deficient MCCs of E17 tracheae.

Double IF analysis of mTEC cultures at early stage of MCC differentiation and centriole amplification (ALI day 3) using antibodies for the deuterosome protein DEUP1 and the early centriole marker SAS-6. Deuterosome numbers are decreased and deuterosome size enlarged in Ccno-deficient MCCs as quantified in (C). Deuterosome-dependent centriole biogenesis is severely reduced in Ccno-deficient MCCs as shown by reduced staining for the early centriole marker SAS-6.
IF staining for DEUP1 reveals a further size increase of deuterosomes of Ccno-deficient MCCs at later stages of centriole biogenesis (ALI day 7). Deuterosome number and size were quantified in (C). IF using a Centrin antibody shows globally reduced presence of centrioles at ALI day 7, which stay localized below the level of deuterosomes and fail to dock to the apical cell membrane in Ccno-deficient MCCs as seen in z-projection.
IF staining for DEUP1 in (A, B) was analysed by Imaris 7.7.2 software to calculate average deuterosome number of wild-type (n = 17) and Ccno-deficient (n = 19) MCCs at ALI day 3, and wild-type (n = 12) and Ccno-deficient (n = 14) MCCs at ALI day 7. Deuterosome size was measured using the spot measurement tool in n = 10 MCCs for both genotypes at ALI day 3, and in wild-type (n = 12) and Ccno-deficient (n = 14) MCCs at ALI day 7.
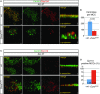
A,B IF analysis of mTEC cultures at ALI day 7 using antibodies against the centriole marker Centrin and CEP164, which marks maturing centrioles. At ALI day 7, centrioles of wild-type MCCs are decorated with CEP164 and are mostly localized to the apical cell membrane. The fewer centrioles of Ccno-deficient MCCs do not colocalize with CEP164 and fail to reach the apical cell surface as seen in z-projection. Lack of CEP164 indicates immature centrioles in Ccno-deficient MCCs. (B) Total centriole numbers per MCC were counted in n = 11 MCCs for both genotypes using Imaris 7.7.2 software.
C,D Double IF staining using Centrin- and CP110-specific antibodies shows an almost complete absence of CP110 from Centrin-positive centrioles in the majority of wild-type MCCs at day 7 of ALI cultures. Most Ccno-deficient MCCs exhibit pronounced CP110 staining that colocalizes with Centrin as seen in z-projection. (D) CP110-positive MCCs were counted in wild-type and Ccno-deficient MTEC cultures and the percentages of CP110-positive MCCs from total MCCs calculated.
References
-
- Al Jord A, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature. 2014;516:104–107. - PubMed
-
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

