An ensemble-averaged, cell density-based digital model of zebrafish embryo development derived from light-sheet microscopy data with single-cell resolution
- PMID: 25712513
- PMCID: PMC5390106
- DOI: 10.1038/srep08601
An ensemble-averaged, cell density-based digital model of zebrafish embryo development derived from light-sheet microscopy data with single-cell resolution
Abstract
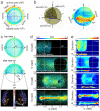
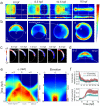
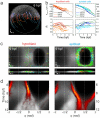
A new era in developmental biology has been ushered in by recent advances in the quantitative imaging of all-cell morphogenesis in living organisms. Here we have developed a light-sheet fluorescence microscopy-based framework with single-cell resolution for identification and characterization of subtle phenotypical changes of millimeter-sized organisms. Such a comparative study requires analyses of entire ensembles to be able to distinguish sample-to-sample variations from definitive phenotypical changes. We present a kinetic digital model of zebrafish embryos up to 16 h of development. The model is based on the precise overlay and averaging of data taken on multiple individuals and describes the cell density and its migration direction at every point in time. Quantitative metrics for multi-sample comparative studies have been introduced to analyze developmental variations within the ensemble. The digital model may serve as a canvas on which the behavior of cellular subpopulations can be studied. As an example, we have investigated cellular rearrangements during germ layer formation at the onset of gastrulation. A comparison of the one-eyed pinhead (oep) mutant with the digital model of the wild-type embryo reveals its abnormal development at the onset of gastrulation, many hours before changes are obvious to the eye.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Keller P. J., Schmidt A. D., Wittbrodt J. & Stelzer E. H. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

