Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist
- PMID: 25712721
- PMCID: PMC4354462
- DOI: 10.1161/HYPERTENSIONAHA.114.05099
Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist
Abstract
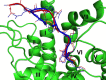
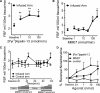
[Pyr(1)]apelin-13 is an endogenous vasodilator and inotrope but is downregulated in pulmonary hypertension and heart failure, making the apelin receptor an attractive therapeutic target. Agonists acting at the same G-protein-coupled receptor can be engineered to stabilize different conformational states and function as biased ligands, selectively stimulating either G-protein or β-arrestin pathways. We used molecular dynamics simulations of apelin/receptor interactions to design cyclic analogues and identified MM07 as a biased agonist. In β-arrestin and internalization assays (G-protein-independent), MM07 was 2 orders of magnitude less potent than [Pyr(1)]apelin-13. In a G-protein-dependent saphenous vein contraction assay, both peptides had comparable potency (pD2:[Pyr(1)]apelin-13 9.93±0.24; MM07 9.54±0.42) and maximum responses with a resulting bias for MM07 of ≈350- to 1300-fold for the G-protein pathway. In rats, systemic infusions of MM07 (10-100nmol) caused a dose-dependent increase in cardiac output that was significantly greater than the response to [Pyr(1)]apelin-13. Similarly, in human volunteers, MM07 produced a significant dose-dependent increase in forearm blood flow with a maximum dilatation double that is seen with [Pyr(1)]apelin-13. Additionally, repeated doses of MM07 produced reproducible increases in forearm blood flow. These responses are consistent with a more efficacious action of the biased agonist. In human hand vein, both peptides reversed an established norepinephrine constrictor response and significantly increased venous flow. Our results suggest that MM07 acting as a biased agonist at the apelin receptor can preferentially stimulate the G-protein pathway, which could translate to improved efficacy in the clinic by selectively stimulating vasodilatation and inotropic actions but avoiding activating detrimental β-arrestin-dependent pathways.
Keywords: [Pyr1]apelin-13; pulmonary arterial hypertension; β-arrestin G-protein coupled receptors.
© 2015 The Authors.
Figures


References
-
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. - PubMed
-
- Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Sharma S, Neilson I, Webb DJ, Megson IL, Flapan AD, Newby DE. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. - PubMed
-
- Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. - PubMed
-
- Salcedo A, Garijo J, Monge L, Fernández N, Luis García-Villalón A, Sánchez Turrión V, Cuervas-Mons V, Diéguez G. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept. 2007;144:50–55. doi: 10.1016/j.regpep.2007.06.005. - PubMed
-
- Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev. 2010;62:331–342. doi: 10.1124/pr.110.002949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

