A Systematic Review and Meta-Analysis of the Seroprevalence of Influenza A(H9N2) Infection Among Humans
- PMID: 25712969
- PMCID: PMC4598807
- DOI: 10.1093/infdis/jiv109
A Systematic Review and Meta-Analysis of the Seroprevalence of Influenza A(H9N2) Infection Among Humans
Abstract
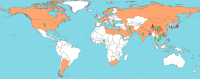
Introduction: Given that influenza A(H9N2) is recognized as a pandemic threat, we evaluated the overall burden of influenza A(H9N2) infections among avian-exposed human populations.
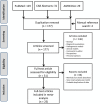
Methods: We performed a systematic search of PubMed, AGRICOLA, and CAB Abstracts databases for literature published during 1997-2013. Studies reporting serological evidence of human influenza A(H9N2) infection among avian-exposed populations were included. We used a World Health Organization (WHO)-recommended case definition for serological evidence of infection based on results of hemagglutination inhibition (HI) and microneutralization (MN) assays. We calculated overall seroprevalence through a random effects meta-analysis model.
Results: Seroprevalence data reported by the studies ranged from 1% to 43% (median, 9%) by HI, which was not significantly different from the seroprevalence estimated through the WHO-recommended case definition (median, 1.3%; range, 0.5%-42.6%). Reported seroprevalence by MN ranged from 0.6% to 9% (median, 2.7%), which was greater than the seroprevalence estimated through the WHO-recommended case definition (median, 0.3%; range, 0.1%-1.4%).
Conclusions: A small proportion of avian-exposed humans had evidence of influenza A(H9N2) infection. As the virus has a near global distribution in poultry, it seems likely that present surveillance efforts are missing mild or asymptomatic infections among avian-exposed persons. It seems prudent to closely monitor avian-exposed populations for influenza A(H9N2) infection to provide prepandemic warnings.
Keywords: H9N2 subtype; hemagglutination inhibition test; influenza A virus; meta-analysis; microneutralization test; seroprevalence; systematic review.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet 1999; 354:916–7. - PubMed
-
- Guo YJ, Krauss S, Senne DA, et al. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 2000; 267:279–88. - PubMed
-
- Guo Y, Li J, Cheng X. Discovery of men infected by avian influenza A (H9N2) virus [in Chinese]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1999; 13:105–8. - PubMed
-
- International Centre for Diarrhoeal Disease Research, Bangladesh/Government of The People's Republic of Bangladesh. Outbreak of mild respiratory disease caused by H5N1 and H9N2 infections among young children in Dhaka, Bangladesh. Health Sci Bull 2011; 9:5–12.
-
- Cheng VC, Chan JF, Wen X, et al. Infection of immunocompromised patients by avian H9N2 influenza A virus. J Infect 2011; 62:394–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

