A triple mutant in the Ω-loop of TEM-1 β-lactamase changes the substrate profile via a large conformational change and an altered general base for catalysis
- PMID: 25713062
- PMCID: PMC4400348
- DOI: 10.1074/jbc.M114.633438
A triple mutant in the Ω-loop of TEM-1 β-lactamase changes the substrate profile via a large conformational change and an altered general base for catalysis
Abstract
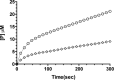
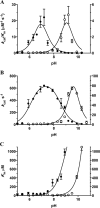
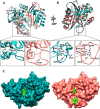
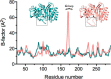
β-Lactamases are bacterial enzymes that hydrolyze β-lactam antibiotics. TEM-1 is a prevalent plasmid-encoded β-lactamase in Gram-negative bacteria that efficiently catalyzes the hydrolysis of penicillins and early cephalosporins but not oxyimino-cephalosporins. A previous random mutagenesis study identified a W165Y/E166Y/P167G triple mutant that displays greatly altered substrate specificity with increased activity for the oxyimino-cephalosporin, ceftazidime, and decreased activity toward all other β-lactams tested. Surprisingly, this mutant lacks the conserved Glu-166 residue critical for enzyme function. Ceftazidime contains a large, bulky side chain that does not fit optimally in the wild-type TEM-1 active site. Therefore, it was hypothesized that the substitutions in the mutant expand the binding site in the enzyme. To investigate structural changes and address whether there is an enlargement in the active site, the crystal structure of the triple mutant was solved to 1.44 Å. The structure reveals a large conformational change of the active site Ω-loop structure to create additional space for the ceftazidime side chain. The position of the hydroxyl group of Tyr-166 and an observed shift in the pH profile of the triple mutant suggests that Tyr-166 participates in the hydrolytic mechanism of the enzyme. These findings indicate that the highly conserved Glu-166 residue can be substituted in the mechanism of serine β-lactamases. The results reveal that the robustness of the overall β-lactamase fold coupled with the plasticity of an active site loop facilitates the evolution of enzyme specificity and mechanism.
Keywords: Antibiotic Resistance; Beta-Lactamase; Enzyme Catalysis; Enzyme Evolution; Enzyme Kinetics; Enzyme Structure; Protein Stability; Protein Structure; X-ray Crystallography.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
The Drug-Resistant Variant P167S Expands the Substrate Profile of CTX-M β-Lactamases for Oxyimino-Cephalosporin Antibiotics by Enlarging the Active Site upon Acylation.Biochemistry. 2017 Jul 11;56(27):3443-3453. doi: 10.1021/acs.biochem.7b00176. Epub 2017 Jun 27. Biochemistry. 2017. PMID: 28613873 Free PMC article.
-
Systematic mutagenesis of the active site omega loop of TEM-1 beta-lactamase.J Bacteriol. 1996 Apr;178(7):1821-8. doi: 10.1128/jb.178.7.1821-1828.1996. J Bacteriol. 1996. PMID: 8606154 Free PMC article.
-
Structural and biochemical evidence that a TEM-1 beta-lactamase N170G active site mutant acts via substrate-assisted catalysis.J Biol Chem. 2009 Nov 27;284(48):33703-12. doi: 10.1074/jbc.M109.053819. Epub 2009 Oct 6. J Biol Chem. 2009. PMID: 19812041 Free PMC article.
-
Structural and Mechanistic Basis for Extended-Spectrum Drug-Resistance Mutations in Altering the Specificity of TEM, CTX-M, and KPC β-lactamases.Front Mol Biosci. 2018 Feb 23;5:16. doi: 10.3389/fmolb.2018.00016. eCollection 2018. Front Mol Biosci. 2018. PMID: 29527530 Free PMC article. Review.
-
Class D β-lactamases: a reappraisal after five decades.Acc Chem Res. 2013 Nov 19;46(11):2407-15. doi: 10.1021/ar300327a. Epub 2013 Jul 31. Acc Chem Res. 2013. PMID: 23902256 Free PMC article. Review.
Cited by
-
An in vivo selection system with tightly regulated gene expression enables directed evolution of highly efficient enzymes.Sci Rep. 2021 Jun 3;11(1):11669. doi: 10.1038/s41598-021-91204-4. Sci Rep. 2021. PMID: 34083677 Free PMC article.
-
The Role of the Ω-Loop in Regulation of the Catalytic Activity of TEM-Type β-Lactamases.Biomolecules. 2019 Dec 11;9(12):854. doi: 10.3390/biom9120854. Biomolecules. 2019. PMID: 31835662 Free PMC article. Review.
-
Removal of the Side Chain at the Active-Site Serine by a Glycine Substitution Increases the Stability of a Wide Range of Serine β-Lactamases by Relieving Steric Strain.Biochemistry. 2016 May 3;55(17):2479-90. doi: 10.1021/acs.biochem.6b00056. Epub 2016 Apr 22. Biochemistry. 2016. PMID: 27073009 Free PMC article.
-
A novel strategy to characterize the pattern of β-lactam antibiotic-induced drug resistance in Acinetobacter baumannii.Res Sq [Preprint]. 2023 Jan 19:rs.3.rs-2359505. doi: 10.21203/rs.3.rs-2359505/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Jun 6;13(1):9177. doi: 10.1038/s41598-023-36475-9. PMID: 36711967 Free PMC article. Updated. Preprint.
-
Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability.PLoS Pathog. 2015 Jun 1;11(6):e1004949. doi: 10.1371/journal.ppat.1004949. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26030609 Free PMC article.
References
-
- Pfeifer Y., Cullik A., Witte W. (2010) Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int. J. Med. Microbiol. 300, 371–379 - PubMed
-
- Ambler R. P. (1980) The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289, 321–331 - PubMed
-
- Page M. I. (1999) The reactivity of β-lactams, the mechanism of catalysis and the inhibition of β-lactamases. Curr. Pharm. Des. 5, 895–913 - PubMed
-
- Hata M., Fujii Y., Tanaka Y., Ishikawa H., Ishii M., Neya S., Tsuda M., Hoshino T. (2006) Substrate deacylation mechanisms of serine-β-lactamases. Biol. Pharm. Bull 29, 2151–2159 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

