Mechanisms of membrane binding of small GTPase K-Ras4B farnesylated hypervariable region
- PMID: 25713064
- PMCID: PMC4392252
- DOI: 10.1074/jbc.M114.620724
Mechanisms of membrane binding of small GTPase K-Ras4B farnesylated hypervariable region
Abstract
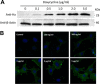
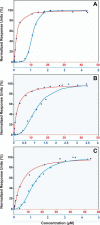
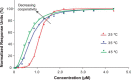
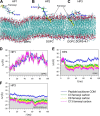
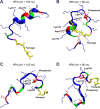
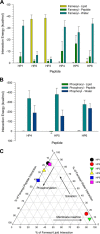
K-Ras4B belongs to a family of small GTPases that regulates cell growth, differentiation and survival. K-ras is frequently mutated in cancer. K-Ras4B association with the plasma membrane through its farnesylated and positively charged C-terminal hypervariable region (HVR) is critical to its oncogenic function. However, the structural mechanisms of membrane association are not fully understood. Here, using confocal microscopy, surface plasmon resonance, and molecular dynamics simulations, we observed that K-Ras4B can be distributed in rigid and loosely packed membrane domains. Its membrane binding domain interaction with phospholipids is driven by membrane fluidity. The farnesyl group spontaneously inserts into the disordered lipid microdomains, whereas the rigid microdomains restrict the farnesyl group penetration. We speculate that the resulting farnesyl protrusion toward the cell interior allows oligomerization of the K-Ras4B membrane binding domain in rigid microdomains. Unlike other Ras isoforms, K-Ras4B HVR contains a single farnesyl modification and positively charged polylysine sequence. The high positive charge not only modulates specific HVR binding to anionic phospholipids but farnesyl membrane orientation. Phosphorylation of Ser-181 prohibits spontaneous farnesyl membrane insertion. The mechanism illuminates the roles of HVR modifications in K-Ras4B targeting microdomains of the plasma membrane and suggests an additional function for HVR in regulation of Ras signaling.
Keywords: Cooperativity; HVR; Membrane Microdomains; Phospholipid; Phosphorylation; Post-translational Modification (PTM); Protein Isoprenylation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Friday B. B., Adjei A. A. (2005) K-ras as a target for cancer therapy. Biochim. Biophys. Acta 1756, 127–144 - PubMed
-
- Ellis C. A., Clark G. (2000) The importance of being K-Ras. Cell. Signal. 12, 425–434 - PubMed
-
- Esteban L. M., Vicario-Abejón C., Fernández-Salguero P., Fernández-Medarde A., Swaminathan N., Yienger K., Lopez E., Malumbres M., McKay R., Ward J. M., Pellicer A., Santos E. (2001) Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol. Cell. Biol. 21, 1444–1452 - PMC - PubMed
-
- Koera K., Nakamura K., Nakao K., Miyoshi J., Toyoshima K., Hatta T., Otani H., Aiba A., Katsuki M. (1997) K-ras is essential for the development of the mouse embryo. Oncogene 15, 1151–1159 - PubMed
-
- Plowman S. J., Williamson D. J., O'Sullivan M. J., Doig J., Ritchie A. M., Harrison D. J., Melton D. W., Arends M. J., Hooper M. L., Patek C. E. (2003) While K-ras is essential for mouse development, expression of the K-ras 4A splice variant is dispensable. Mol. Cell. Biol. 23, 9245–9250 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

