Fo-driven Rotation in the ATP Synthase Direction against the Force of F1 ATPase in the FoF1 ATP Synthase
- PMID: 25713065
- PMCID: PMC4409238
- DOI: 10.1074/jbc.M115.646430
Fo-driven Rotation in the ATP Synthase Direction against the Force of F1 ATPase in the FoF1 ATP Synthase
Abstract
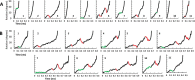
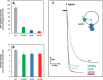
Living organisms rely on the FoF1 ATP synthase to maintain the non-equilibrium chemical gradient of ATP to ADP and phosphate that provides the primary energy source for cellular processes. How the Fo motor uses a transmembrane electrochemical ion gradient to create clockwise torque that overcomes F1 ATPase-driven counterclockwise torque at high ATP is a major unresolved question. Using single FoF1 molecules embedded in lipid bilayer nanodiscs, we now report the observation of Fo-dependent rotation of the c10 ring in the ATP synthase (clockwise) direction against the counterclockwise force of ATPase-driven rotation that occurs upon formation of a leash with Fo stator subunit a. Mutational studies indicate that the leash is important for ATP synthase activity and support a mechanism in which residues aGlu-196 and cArg-50 participate in the cytoplasmic proton half-channel to promote leash formation.
Keywords: ATP Synthase; F1Fo ATPase; Molecular Motor; Nanodiscs; Proton Transport; Single-molecule Biophysics.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Spetzler D., Ishmukhametov R., Hornung T., Martin J., York J., Jin-Day L., Frasch W. D. (eds) (2012) Photosynthesis, in Advances in Photosynthesis and Respiration (Eaton-Rye J., Tripathy B., Sharkey T., eds.), Vol. 34, pp. 561–590, Springer, Dordrecht, The Netherlands
-
- Girvin M. E., Fillingame R. H. (1994) Hairpin folding of subunit c of F1Fo ATP synthase: 1H distance measurements to nitroxide-derivatized aspartyl-61. Biochemistry 33, 665–674 - PubMed
-
- Pogoryelov D., Yildiz O., Faraldo-Gómez J. D., Meier T. (2009) High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat. Struct. Mol. Biol. 16, 1068–1073 - PubMed
-
- Pogoryelov D., Krah A., Langer J. D., Yildiz Ö., Faraldo-Gómez J. D., Meier T. (2010) Microscopic rotary mechanism of ion translocation in the F-0 complex of ATP synthases. Nat. Chem. Biol. 6, 891–899 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

