Rescue of Na+ affinity in aspartate 928 mutants of Na+,K+-ATPase by secondary mutation of glutamate 314
- PMID: 25713066
- PMCID: PMC4392278
- DOI: 10.1074/jbc.M114.625509
Rescue of Na+ affinity in aspartate 928 mutants of Na+,K+-ATPase by secondary mutation of glutamate 314
Abstract
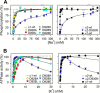
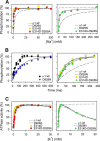
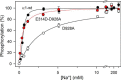
The Na(+),K(+)-ATPase binds Na(+) at three transport sites denoted I, II, and III, of which site III is Na(+)-specific and suggested to be the first occupied in the cooperative binding process activating phosphorylation from ATP. Here we demonstrate that the asparagine substitution of the aspartate associated with site III found in patients with rapid-onset dystonia parkinsonism or alternating hemiplegia of childhood causes a dramatic reduction of Na(+) affinity in the α1-, α2-, and α3-isoforms of Na(+),K(+)-ATPase, whereas other substitutions of this aspartate are much less disruptive. This is likely due to interference by the amide function of the asparagine side chain with Na(+)-coordinating residues in site III. Remarkably, the Na(+) affinity of site III aspartate to asparagine and alanine mutants is rescued by second-site mutation of a glutamate in the extracellular part of the fourth transmembrane helix, distant to site III. This gain-of-function mutation works without recovery of the lost cooperativity and selectivity of Na(+) binding and does not affect the E1-E2 conformational equilibrium or the maximum phosphorylation rate. Hence, the rescue of Na(+) affinity is likely intrinsic to the Na(+) binding pocket, and the underlying mechanism could be a tightening of Na(+) binding at Na(+) site II, possibly via movement of transmembrane helix four. The second-site mutation also improves Na(+),K(+) pump function in intact cells. Rescue of Na(+) affinity and Na(+) and K(+) transport by second-site mutation is unique in the history of Na(+),K(+)-ATPase and points to new possibilities for treatment of neurological patients carrying Na(+),K(+)-ATPase mutations.
Keywords: Alternating Hemiplegia of Childhood; Membrane Transport; Na+,K+ Pump; Na+/K+-ATPase; Neurological Disease; P-type ATPase; Rapid-onset Dystonia Parkinsonism; Second-site Revertant; Site-directed Mutagenesis; Sodium Transport.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures








Similar articles
-
Relationship between intracellular Na+ concentration and reduced Na+ affinity in Na+,K+-ATPase mutants causing neurological disease.J Biol Chem. 2014 Feb 7;289(6):3186-97. doi: 10.1074/jbc.M113.543272. Epub 2013 Dec 19. J Biol Chem. 2014. PMID: 24356962 Free PMC article.
-
Distinct effects of Q925 mutation on intracellular and extracellular Na+ and K+ binding to the Na+, K+-ATPase.Sci Rep. 2019 Sep 16;9(1):13344. doi: 10.1038/s41598-019-50009-2. Sci Rep. 2019. PMID: 31527711 Free PMC article.
-
Substitutions of glutamate 781 in the Na,K-ATPase alpha subunit demonstrate reduced cation selectivity and an increased affinity for ATP.J Biol Chem. 1996 Feb 2;271(5):2413-21. doi: 10.1074/jbc.271.5.2413. J Biol Chem. 1996. PMID: 8576200
-
Neurological disease mutations of α3 Na+,K+-ATPase: Structural and functional perspectives and rescue of compromised function.Biochim Biophys Acta. 2016 Nov;1857(11):1807-1828. doi: 10.1016/j.bbabio.2016.08.009. Epub 2016 Aug 28. Biochim Biophys Acta. 2016. PMID: 27577505 Review.
-
Structure-function relationships of Na(+), K(+), ATP, or Mg(2+) binding and energy transduction in Na,K-ATPase.Biochim Biophys Acta. 2001 May 1;1505(1):57-74. doi: 10.1016/s0005-2728(00)00277-2. Biochim Biophys Acta. 2001. PMID: 11248189 Review.
Cited by
-
D-DEMØ, a distinct phenotype caused by ATP1A3 mutations.Neurol Genet. 2020 Aug 4;6(5):e466. doi: 10.1212/NXG.0000000000000466. eCollection 2020 Oct. Neurol Genet. 2020. PMID: 32802951 Free PMC article.
-
Distinct pH dependencies of Na+/K+ selectivity at the two faces of Na,K-ATPase.J Biol Chem. 2018 Feb 9;293(6):2195-2205. doi: 10.1074/jbc.RA117.000700. Epub 2017 Dec 15. J Biol Chem. 2018. PMID: 29247005 Free PMC article.
-
Functional consequences of the CAPOS mutation E818K of Na+,K+-ATPase.J Biol Chem. 2019 Jan 4;294(1):269-280. doi: 10.1074/jbc.RA118.004591. Epub 2018 Nov 8. J Biol Chem. 2019. PMID: 30409907 Free PMC article.
-
Rapid-Onset Dystonia-Parkinsonism Phenotype Consistency for a Novel Variant of ATP1A3 in Patients Across 3 Global Populations.Neurol Genet. 2021 Mar 15;7(2):e562. doi: 10.1212/NXG.0000000000000562. eCollection 2021 Apr. Neurol Genet. 2021. PMID: 33977143 Free PMC article. No abstract available.
-
Importance of a Potential Protein Kinase A Phosphorylation Site of Na+,K+-ATPase and Its Interaction Network for Na+ Binding.J Biol Chem. 2016 May 13;291(20):10934-47. doi: 10.1074/jbc.M115.701201. Epub 2016 Mar 24. J Biol Chem. 2016. PMID: 27013656 Free PMC article.
References
-
- Post R. L., Hegyvary C., Kume S. (1972) Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247, 6530–6540 - PubMed
-
- Albers R. W., (1967) Biochemical aspects of active transport. Annu. Rev. Biochem. 36, 727–756 - PubMed
-
- Kanai R., Ogawa H., Vilsen B., Cornelius F., Toyoshima C. (2013) Crystal structure of a Na-bound Na,K-ATPase preceding the E1P state. Nature 502, 201–206 - PubMed
-
- Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. (2007) Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049 - PubMed
-
- Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Crystal structure of the sodium-potassium pump at 2.4 Ä resolution. Nature 459, 446–450 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

