Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction
- PMID: 25713069
- PMCID: PMC4423682
- DOI: 10.1074/jbc.M114.621003
Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction
Abstract
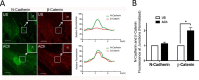
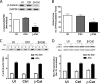
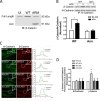
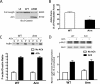
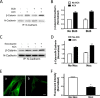
β-Catenin is a key component that connects transmembrane cadherin with the actin cytoskeleton at the cell-cell interface. However, the role of the β-catenin/cadherin interaction in smooth muscle has not been well characterized. Here stimulation with acetylcholine promoted the recruitment of β-catenin to N-cadherin in smooth muscle cells/tissues. Knockdown of β-catenin by lentivirus-mediated shRNA attenuated smooth muscle contraction. Nevertheless, myosin light chain phosphorylation at Ser-19 and actin polymerization in response to contractile activation were not reduced by β-catenin knockdown. In addition, the expression of the β-catenin armadillo domain disrupted the recruitment of β-catenin to N-cadherin. Force development, but not myosin light chain phosphorylation and actin polymerization, was reduced by the expression of the β-catenin armadillo domain. Furthermore, actin polymerization and microtubules have been implicated in intracellular trafficking. In this study, the treatment with the inhibitor latrunculin A diminished the interaction of β-catenin with N-cadherin in smooth muscle. In contrast, the exposure of smooth muscle to the microtubule depolymerizer nocodazole did not affect the protein-protein interaction. Together, these findings suggest that smooth muscle contraction is mediated by the recruitment of β-catenin to N-cadherin, which may facilitate intercellular mechanotransduction. The association of β-catenin with N-cadherin is regulated by actin polymerization during contractile activation.
Keywords: Adherens Junction; Cytoskeleton; Excitation-Contraction Coupling (E-C Coupling); Signal Transduction; Smooth Muscle.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

