Alcohol inhibits osteopontin-dependent transforming growth factor-β1 expression in human mesenchymal stem cells
- PMID: 25713073
- PMCID: PMC4400371
- DOI: 10.1074/jbc.M114.616888
Alcohol inhibits osteopontin-dependent transforming growth factor-β1 expression in human mesenchymal stem cells
Abstract
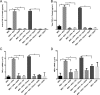
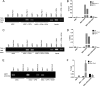
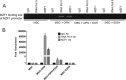
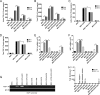
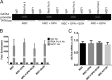
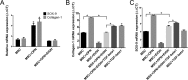
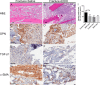
Alcohol (EtOH) intoxication is a risk factor for increased morbidity and mortality with traumatic injuries, in part through inhibition of bone fracture healing. Animal models have shown that EtOH decreases fracture callus volume, diameter, and biomechanical strength. Transforming growth factor β1 (TGF-β1) and osteopontin (OPN) play important roles in bone remodeling and fracture healing. Mesenchymal stem cells (MSC) reside in bone and are recruited to fracture sites for the healing process. Resident MSC are critical for fracture healing and function as a source of TGF-β1 induced by local OPN, which acts through the transcription factor myeloid zinc finger 1 (MZF1). The molecular mechanisms responsible for the effect of EtOH on fracture healing are still incompletely understood, and this study investigated the role of EtOH in affecting OPN-dependent TGF-β1 expression in MSC. We have demonstrated that EtOH inhibits OPN-induced TGF-β1 protein expression, decreases MZF1-dependent TGF-β1 transcription and MZF1 transcription, and blocks OPN-induced MZF1 phosphorylation. We also found that PKA signaling enhances OPN-induced TGF-β1 expression. Last, we showed that EtOH exposure reduces the TGF-β1 protein levels in mouse fracture callus. We conclude that EtOH acts in a novel mechanism by interfering directly with the OPN-MZF1-TGF-β1 signaling pathway in MSC.
Keywords: Alcohol (EtOH); Gene Expression; Gene Transcription; Mesenchymal Stem Cells (MSCs); Osteopontin; Promoter; Protein Kinase A (PKA); Transforming Growth Factor β (TGF-β); Zinc Finger.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


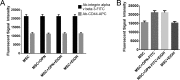
References
-
- Rivara F. P., Jurkovich G. J., Gurney J. G., Seguin D., Fligner C. L., Ries R., Raisys V. A., Copass M. (1993) The magnitude of acute and chronic alcohol abuse in trauma patients. Arch. Surg. 128, 907–912; discussion 912–903 - PubMed
-
- Demetriades D., Gkiokas G., Velmahos G. C., Brown C., Murray J., Noguchi T. (2004) Alcohol and illicit drugs in traumatic deaths: prevalence and association with type and severity of injuries. J. Am. Coll. Surg. 199, 687–692 - PubMed
-
- Nyquist F., Berglund M., Nilsson B. E., Obrant K. J. (1997) Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol. 32, 91–95 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

