The pivotal role of the mitochondrial amidoxime reducing component 2 in protecting human cells against apoptotic effects of the base analog N6-hydroxylaminopurine
- PMID: 25713076
- PMCID: PMC4400328
- DOI: 10.1074/jbc.M115.640052
The pivotal role of the mitochondrial amidoxime reducing component 2 in protecting human cells against apoptotic effects of the base analog N6-hydroxylaminopurine
Abstract
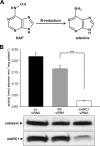
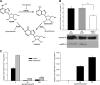
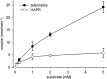
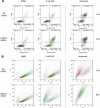
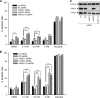
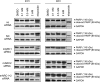
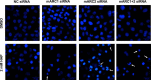
N-Hydroxylated nucleobases and nucleosides as N-hydroxylaminopurine (HAP) or N-hydroxyadenosine (HAPR) may be generated endogenously in the course of cell metabolism by cytochrome P450, by oxidative stress or by a deviating nucleotide biosynthesis. These compounds have shown to be toxic and mutagenic for procaryotic and eucaryotic cells. For DNA replication fidelity it is therefore of great importance that organisms exhibit effective mechanisms to remove such non-canonical base analogs from DNA precursor pools. In vitro, the molybdoenzymes mitochondrial amidoxime reducing component 1 and 2 (mARC1 and mARC2) have shown to be capable of reducing N-hydroxylated base analogs and nucleoside analogs to the corresponding canonical nucleobases and nucleosides upon reconstitution with the electron transport proteins cytochrome b5 and NADH-cytochrome b5 reductase. By RNAi-mediated down-regulation of mARC in human cell lines the mARC-dependent N-reductive detoxication of HAP in cell metabolism could be demonstrated. For HAPR, on the other hand, the reduction to adenosine seems to be of less significance in the detoxication pathway of human cells as HAPR is primarily metabolized to inosine by direct dehydroxylamination catalyzed by adenosine deaminase. Furthermore, the effect of mARC knockdown on sensitivity of human cells to HAP was examined by flow cytometric quantification of apoptotic cell death and detection of poly (ADP-ribose) polymerase (PARP) cleavage. mARC2 was shown to protect HeLa cells against the apoptotic effects of the base analog, whereas the involvement of mARC1 in reductive detoxication of HAP does not seem to be pivotal.
Keywords: MOSC; N-reduction; N6-hydroxyadenosine; N6-hydroxylaminopurine; RNA interference (RNAi); apoptosis; cell metabolism; flow cytometry; mARC; nucleoside/nucleotide analogue.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
The involvement of mitochondrial amidoxime reducing components 1 and 2 and mitochondrial cytochrome b5 in N-reductive metabolism in human cells.J Biol Chem. 2013 Jul 12;288(28):20228-37. doi: 10.1074/jbc.M113.474916. Epub 2013 May 23. J Biol Chem. 2013. PMID: 23703616 Free PMC article.
-
Reduction of sulfamethoxazole hydroxylamine (SMX-HA) by the mitochondrial amidoxime reducing component (mARC).Chem Res Toxicol. 2014 Oct 20;27(10):1687-95. doi: 10.1021/tx500174u. Epub 2014 Sep 18. Chem Res Toxicol. 2014. PMID: 25170804
-
The mammalian molybdenum enzymes of mARC.J Biol Inorg Chem. 2015 Mar;20(2):265-75. doi: 10.1007/s00775-014-1216-4. Epub 2014 Nov 26. J Biol Inorg Chem. 2015. PMID: 25425164 Review.
-
The mitochondrial Amidoxime Reducing Component (mARC) is involved in detoxification of N-hydroxylated base analogues.Chem Res Toxicol. 2012 Nov 19;25(11):2443-50. doi: 10.1021/tx300298m. Epub 2012 Aug 27. Chem Res Toxicol. 2012. PMID: 22924387
-
The fourth mammalian molybdenum enzyme mARC: current state of research.Drug Metab Rev. 2011 Nov;43(4):524-39. doi: 10.3109/03602532.2011.608682. Epub 2011 Sep 26. Drug Metab Rev. 2011. PMID: 21942410 Review.
Cited by
-
Expression and Function of mARC: Roles in Lipogenesis and Metabolic Activation of Ximelagatran.PLoS One. 2015 Sep 17;10(9):e0138487. doi: 10.1371/journal.pone.0138487. eCollection 2015. PLoS One. 2015. PMID: 26378779 Free PMC article.
-
A commensal strain of Staphylococcus epidermidis protects against skin neoplasia.Sci Adv. 2018 Feb 28;4(2):eaao4502. doi: 10.1126/sciadv.aao4502. eCollection 2018 Feb. Sci Adv. 2018. PMID: 29507878 Free PMC article.
-
High Antitumor Activity of the Dual Topoisomerase Inhibitor P8-D6 in Breast Cancer.Cancers (Basel). 2021 Dec 21;14(1):2. doi: 10.3390/cancers14010002. Cancers (Basel). 2021. PMID: 35008166 Free PMC article.
-
Molybdenum's Role as an Essential Element in Enzymes Catabolizing Redox Reactions: A Review.Biomolecules. 2024 Jul 19;14(7):869. doi: 10.3390/biom14070869. Biomolecules. 2024. PMID: 39062583 Free PMC article. Review.
-
Newly developed dual topoisomerase inhibitor P8-D6 is highly active in ovarian cancer.Ther Adv Med Oncol. 2021 Nov 25;13:17588359211059896. doi: 10.1177/17588359211059896. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34887943 Free PMC article.
References
-
- Khromov-Borisov N. N. (1997) Naming the mutagenic nucleic acid base analogs: the Galatea syndrome. Mutat. Res. 379, 95–103 - PubMed
-
- Negishi K., Bessho T., Hayatsu H. (1994) Nucleoside and nucleobase analog mutagens. Mutat. Res 318, 227–238 - PubMed
-
- Freese E. (1959) The specific mutagenic effect of base analogues on Phage T4. J. Mol. Biol. 1, 87–105
-
- Kozmin S. G., Schaaper R. M., Shcherbakova P. V., Kulikov V. N., Noskov V. N., Guetsova M. L., Alenin V. V., Rogozin I. B., Makarova K. S., Pavlov Y. I. (1998) Multiple antimutagenesis mechanisms affect mutagenic activity and specificity of the base analog 6-N-hydroxylaminopurine in bacteria and yeast. Mutat. Res. 402, 41–50 - PubMed
-
- Krenitsky T. A., Neil S. M., Elion G. B., Hitchings G. H. (1969) Adenine phosphoribosyltransferase from monkey liver. Specificity and properties. J. Biol. Chem. 244, 4779–4784 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

