Histone H2A and H4 N-terminal tails are positioned by the MEP50 WD repeat protein for efficient methylation by the PRMT5 arginine methyltransferase
- PMID: 25713080
- PMCID: PMC4392268
- DOI: 10.1074/jbc.M115.636894
Histone H2A and H4 N-terminal tails are positioned by the MEP50 WD repeat protein for efficient methylation by the PRMT5 arginine methyltransferase
Abstract
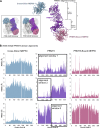
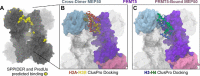
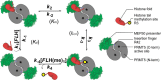
The protein arginine methyltransferase PRMT5 is complexed with the WD repeat protein MEP50 (also known as Wdr77 or androgen coactivator p44) in vertebrates in a tetramer of heterodimers. MEP50 is hypothesized to be required for protein substrate recruitment to the catalytic domain of PRMT5. Here we demonstrate that the cross-dimer MEP50 is paired with its cognate PRMT5 molecule to promote histone methylation. We employed qualitative methylation assays and a novel ultrasensitive continuous assay to measure enzyme kinetics. We demonstrate that neither full-length human PRMT5 nor the Xenopus laevis PRMT5 catalytic domain has appreciable protein methyltransferase activity. We show that histones H4 and H3 bind PRMT5-MEP50 more efficiently compared with histone H2A(1-20) and H4(1-20) peptides. Histone binding is mediated through histone fold interactions as determined by competition experiments and by high density histone peptide array interaction studies. Nucleosomes are not a substrate for PRMT5-MEP50, consistent with the primary mode of interaction via the histone fold of H3-H4, obscured by DNA in the nucleosome. Mutation of a conserved arginine (Arg-42) on the MEP50 insertion loop impaired the PRMT5-MEP50 enzymatic efficiency by increasing its histone substrate Km, comparable with that of Caenorhabditis elegans PRMT5. We show that PRMT5-MEP50 prefers unmethylated substrates, consistent with a distributive model for dimethylation and suggesting discrete biological roles for mono- and dimethylarginine-modified proteins. We propose a model in which MEP50 and PRMT5 simultaneously engage the protein substrate, orienting its targeted arginine to the catalytic site.
Keywords: Enzyme Kinetics; Enzyme Mechanism; Histone Methylation; Peptide Array; Protein Arginine N-methyltransferase 5 (PRMT5); WD Repeat.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Wysocka J., Allis C. D., Coonrod S. (2006) Histone arginine methylation and its dynamic regulation. Front. Biosci. 11, 344–355 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

