Golgi anti-apoptotic proteins are highly conserved ion channels that affect apoptosis and cell migration
- PMID: 25713081
- PMCID: PMC4416878
- DOI: 10.1074/jbc.M115.637306
Golgi anti-apoptotic proteins are highly conserved ion channels that affect apoptosis and cell migration
Abstract
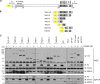
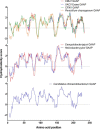
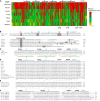
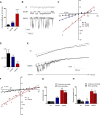
Golgi anti-apoptotic proteins (GAAPs) are multitransmembrane proteins that are expressed in the Golgi apparatus and are able to homo-oligomerize. They are highly conserved throughout eukaryotes and are present in some prokaryotes and orthopoxviruses. Within eukaryotes, GAAPs regulate the Ca(2+) content of intracellular stores, inhibit apoptosis, and promote cell adhesion and migration. Data presented here demonstrate that purified viral GAAPs (vGAAPs) and human Bax inhibitor 1 form ion channels and that vGAAP from camelpox virus is selective for cations. Mutagenesis of vGAAP, including some residues conserved in the recently solved structure of a related bacterial protein, BsYetJ, altered the conductance (E207Q and D219N) and ion selectivity (E207Q) of the channel. Mutation of residue Glu-207 or -178 reduced the effects of GAAP on cell migration and adhesion without affecting protection from apoptosis. In contrast, mutation of Asp-219 abrogated the anti-apoptotic activity of GAAP but not its effects on cell migration and adhesion. These results demonstrate that GAAPs are ion channels and define residues that contribute to the ion-conducting pore and affect apoptosis, cell adhesion, and migration independently.
Keywords: Electrophysiology; Ion Channel; Lipid Bilayer; Membrane Protein; Viral Protein.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Hu L., Smith T. F., Goldberger G. (2009) LFG: a candidate apoptosis regulatory gene family. Apoptosis 14, 1255–1265 - PubMed
-
- Mariotti M., Smith T. F., Sudmant P. H., Goldberger G. (2014) Pseudogenization of testis-specific Lfg5 predates human/Neanderthal divergence. J. Hum. Genet. 59, 288–291 - PubMed
-
- Lee S., Jo M., Lee J., Koh S. S., Kim S. (2007) Identification of novel universal housekeeping genes by statistical analysis of microarray data. J. Biochem. Mol. Biol. 40, 226–231 - PubMed
-
- Gubser C., Smith G. L. (2002) The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 83, 855–872 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

