Thermodynamic and kinetic characterization of the protein Z-dependent protease inhibitor (ZPI)-protein Z interaction reveals an unexpected role for ZPI Lys-239
- PMID: 25713144
- PMCID: PMC4392287
- DOI: 10.1074/jbc.M114.633479
Thermodynamic and kinetic characterization of the protein Z-dependent protease inhibitor (ZPI)-protein Z interaction reveals an unexpected role for ZPI Lys-239
Abstract
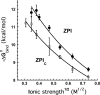
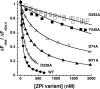
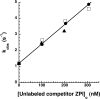
The anticoagulant serpin, protein Z-dependent protease inhibitor (ZPI), circulates in blood as a tight complex with its cofactor, protein Z (PZ), enabling it to function as a rapid inhibitor of membrane-associated factor Xa. Here, we show that N,N'-dimethyl-N-(acetyl)-N'-(7-nitrobenz-3-oxa-1,3-diazol-4-yl)ethylenediamine (NBD)-fluorophore-labeled K239C ZPI is a sensitive, moderately perturbing reporter of the ZPI-PZ interaction and utilize the labeled ZPI to characterize in-depth the thermodynamics and kinetics of wild-type and variant ZPI-PZ interactions. NBD-labeled K239C ZPI bound PZ with ∼3 nM KD and ∼400% fluorescence enhancement at physiologic pH and ionic strength. The NBD-ZPI-PZ interaction was markedly sensitive to ionic strength and pH but minimally affected by temperature, consistent with the importance of charged interactions. NBD-ZPI-PZ affinity was reduced ∼5-fold by physiologic calcium levels to resemble NBD-ZPI affinity for γ-carboxyglutamic acid/EGF1-domainless PZ. Competitive binding studies with ZPI variants revealed that in addition to previously identified Asp-293 and Tyr-240 hot spot residues, Met-71, Asp-74, and Asp-238 made significant contributions to PZ binding, whereas Lys-239 antagonized binding. Rapid kinetic studies indicated a multistep binding mechanism with diffusion-limited association and slow complex dissociation. ZPI complexation with factor Xa or cleavage decreased ZPI-PZ affinity 2-7-fold by increasing the rate of PZ dissociation. A catalytic role for PZ was supported by the correlation between a decreased rate of PZ dissociation from the K239A ZPI-PZ complex and an impaired ability of PZ to catalyze the K239A ZPI-factor Xa reaction. Together, these results reveal the energetic basis of the ZPI-PZ interaction and suggest an important role for ZPI Lys-239 in PZ catalytic action.
Keywords: Anticoagulant; Coagulation Factor; Factor Xa; Kinetics; Protease; Protease Inhibitor; Protein Z; Serpin; ZPI.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







Similar articles
-
Basis for the specificity and activation of the serpin protein Z-dependent proteinase inhibitor (ZPI) as an inhibitor of membrane-associated factor Xa.J Biol Chem. 2010 Jun 25;285(26):20399-409. doi: 10.1074/jbc.M110.112748. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427285 Free PMC article.
-
Characterization of the protein Z-dependent protease inhibitor interactive-sites of protein Z.Biochim Biophys Acta. 2014 Sep;1844(9):1631-7. doi: 10.1016/j.bbapap.2014.06.011. Epub 2014 Jun 21. Biochim Biophys Acta. 2014. PMID: 24960590 Free PMC article.
-
Kinetic characterization of the protein Z-dependent protease inhibitor reaction with blood coagulation factor Xa.J Biol Chem. 2008 Oct 31;283(44):29770-83. doi: 10.1074/jbc.M805214200. Epub 2008 Sep 3. J Biol Chem. 2008. PMID: 18768472 Free PMC article.
-
Protein Z-dependent regulation of coagulation.Thromb Haemost. 2001 Jul;86(1):8-13. Thromb Haemost. 2001. PMID: 11487045 Review.
-
Protein Z, an anticoagulant protein with expanding role in reproductive biology.Reproduction. 2013 Jun 27;146(2):R73-80. doi: 10.1530/REP-13-0072. Print 2013 Aug. Reproduction. 2013. PMID: 23690629 Review.
Cited by
-
Phosphatidylserine and Phosphatidylethanolamine Bind to Protein Z Cooperatively and with Equal Affinity.PLoS One. 2016 Sep 1;11(9):e0161896. doi: 10.1371/journal.pone.0161896. eCollection 2016. PLoS One. 2016. PMID: 27584039 Free PMC article.
-
Engineering a protein Z-dependent protease inhibitor (ZPI) mutant as a novel antagonist of ZPI anticoagulant function for hemophilia treatment.J Thromb Haemost. 2019 Oct;17(10):1655-1660. doi: 10.1111/jth.14610. Epub 2019 Sep 5. J Thromb Haemost. 2019. PMID: 31423718 Free PMC article.
-
Inhibitory serpins. New insights into their folding, polymerization, regulation and clearance.Biochem J. 2016 Aug 1;473(15):2273-93. doi: 10.1042/BCJ20160014. Biochem J. 2016. PMID: 27470592 Free PMC article. Review.
-
Protein Z-dependent protease inhibitor (ZPI) is a physiologically significant inhibitor of prothrombinase function.J Biol Chem. 2019 May 10;294(19):7644-7657. doi: 10.1074/jbc.RA118.006787. Epub 2019 Mar 27. J Biol Chem. 2019. PMID: 30918026 Free PMC article.
-
Tapping Into the Natural PZ-Independent Anticoagulant Function of ZPI to Inhibit Thrombosis With Minimal Effect on Hemostasis.Arterioscler Thromb Vasc Biol. 2025 May;45(5):805-819. doi: 10.1161/ATVBAHA.124.321329. Epub 2025 Feb 20. Arterioscler Thromb Vasc Biol. 2025. PMID: 39973748
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

