Cognitive outcomes after neonatal encephalopathy
- PMID: 25713280
- PMCID: PMC4338321
- DOI: 10.1542/peds.2014-1566
Cognitive outcomes after neonatal encephalopathy
Abstract
Objectives: To describe the spectrum of cognitive outcomes of children with and without cerebral palsy (CP) after neonatal encephalopathy, evaluate the prognostic value of early developmental testing and report on school services and additional therapies.
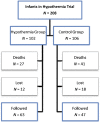
Methods: The participants of this study are the school-aged survivors of the National Institute of Child Health and Human Development Neonatal Research Network randomized controlled trial of whole-body hypothermia. Children underwent neurologic examinations and neurodevelopmental and cognitive testing with the Bayley Scales of Infant Development-II at 18 to 22 months and the Wechsler intelligence scales and the Neuropsychological Assessment-Developmental Neuropsychological Assessment at 6 to 7 years. Parents were interviewed about functional status and receipt of school and support services. We explored predictors of cognitive outcome by using multiple regression models.
Results: Subnormal IQ scores were identified in more than a quarter of the children: 96% of survivors with CP had an IQ <70, 9% of children without CP had an IQ <70, and 31% had an IQ of 70 to 84. Children with a mental developmental index <70 at 18 months had, on average, an adjusted IQ at 6 to 7 years that was 42 points lower than that of those with a mental developmental index >84 (95% confidence interval, -49.3 to -35.0; P < .001). Twenty percent of children with normal IQ and 28% of those with IQ scores of 70 to 84 received special educational support services or were held back ≥1 grade level.
Conclusions: Cognitive impairment remains an important concern for all children with neonatal encephalopathy.
Keywords: cognitive outcomes; hypoxic ischemic encephalopathy; neonatal encephalopathy.
Copyright © 2015 by the American Academy of Pediatrics.
Figures
References
-
- Gluckman PD, Wyatt JS, Azzopardi D, et al. . Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670 - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584 - PubMed
-
- Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group . Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358 - PubMed
-
- Jacobs SE, Morley CJ, Inder TE, et al. Infant Cooling Evaluation Collaboration . Whole-body hypothermia for term and near-term newborns with hypoxic–ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700 - PubMed
-
- Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.network Trial Participants . Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4). Available at: www.pediatrics.org/cgi/content/full/126/4/e771 - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HD027856/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U10 HD021385/HD/NICHD NIH HHS/United States
- U10 HD021364/HD/NICHD NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- U10 HD040521/HD/NICHD NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- M01 RR008084/RR/NCRR NIH HHS/United States
- U10 HD040461/HD/NICHD NIH HHS/United States
- M01 RR016587/RR/NCRR NIH HHS/United States
- U10 HD040689/HD/NICHD NIH HHS/United States
- U10 HD040492/HD/NICHD NIH HHS/United States
- U10 HD027853/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- U10 HD021397/HD/NICHD NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- U10 HD027871/HD/NICHD NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- U10 HD027851/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- U10 HD034216/HD/NICHD NIH HHS/United States
- U10 HD036790/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous