International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors--family B G protein-coupled receptors
- PMID: 25713287
- PMCID: PMC4394688
- DOI: 10.1124/pr.114.009464
International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors--family B G protein-coupled receptors
Abstract
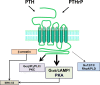
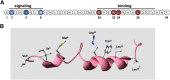
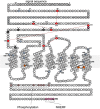
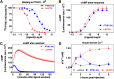
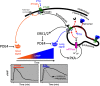
The type-1 parathyroid hormone receptor (PTHR1) is a family B G protein-coupled receptor (GPCR) that mediates the actions of two polypeptide ligands; parathyroid hormone (PTH), an endocrine hormone that regulates the levels of calcium and inorganic phosphate in the blood by acting on bone and kidney, and PTH-related protein (PTHrP), a paracrine-factor that regulates cell differentiation and proliferation programs in developing bone and other tissues. The type-2 parathyroid hormone receptor (PTHR2) binds a peptide ligand, called tuberoinfundibular peptide-39 (TIP39), and while the biologic role of the PTHR2/TIP39 system is not as defined as that of the PTHR1, it likely plays a role in the central nervous system as well as in spermatogenesis. Mechanisms of action at these receptors have been explored through a variety of pharmacological and biochemical approaches, and the data obtained support a basic "two-site" mode of ligand binding now thought to be used by each of the family B peptide hormone GPCRs. Recent crystallographic studies on the family B GPCRs are providing new insights that help to further refine the specifics of the overall receptor architecture and modes of ligand docking. One intriguing pharmacological finding for the PTHR1 is that it can form surprisingly stable complexes with certain PTH/PTHrP ligand analogs and thereby mediate markedly prolonged cell signaling responses that persist even when the bulk of the complexes are found in internalized vesicles. The PTHR1 thus appears to be able to activate the Gα(s)/cAMP pathway not only from the plasma membrane but also from the endosomal domain. The cumulative findings could have an impact on efforts to develop new drug therapies for the PTH receptors.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




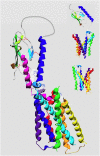
References
-
- Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Jr, et al. (1992) Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89:2732–2736. - PMC - PubMed
-
- Adams AE, Bisello A, Chorev M, Rosenblatt M, Suva LJ. (1998) Arginine 186 in the extracellular N-terminal region of the human parathyroid hormone 1 receptor is essential for contact with position 13 of the hormone. Mol Endocrinol 12:1673–1683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

