Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice
- PMID: 25713289
- PMCID: PMC4345879
- DOI: 10.1161/JAHA.115.001770
Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice
Abstract
Background: Cardiac dysfunction developing in response to chronic pressure overload is associated with apoptotic cell death and myocardial vessel rarefaction. We examined whether deletion of tumor suppressor p53 in endothelial cells may prevent the transition from cardiac hypertrophy to heart failure.
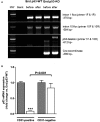
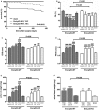
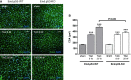
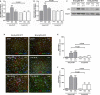
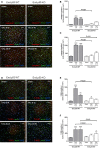
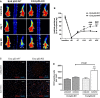
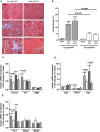
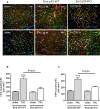
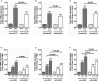
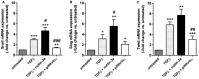
Methods and results: Mice with endothelial-specific deletion of p53 (End.p53-KO) were generated by crossing p53fl/fl mice with mice expressing Cre recombinase under control of an inducible Tie2 promoter. Cardiac hypertrophy was induced by transverse aortic constriction. Serial echocardiography measurements revealed improved cardiac function in End.p53-KO mice that also exhibited better survival. Cardiac hypertrophy was associated with increased p53 levels in End.p53-WT controls, whereas banded hearts of End.p53-KO mice exhibited lower numbers of apoptotic endothelial and non-endothelial cells and altered mRNA levels of genes regulating cell cycle progression (p21), apoptosis (Puma), or proliferation (Pcna). A higher cardiac capillary density and improved myocardial perfusion was observed, and pharmacological inhibition or genetic deletion of p53 also promoted endothelial sprouting in vitro and new vessel formation following hindlimb ischemia in vivo. Hearts of End.p53-KO mice exhibited markedly less fibrosis compared with End.p53-WT controls, and lower mRNA levels of p53-regulated genes involved in extracellular matrix production and turnover (eg, Bmp-7, Ctgf, or Pai-1), or of transcription factors involved in controlling mesenchymal differentiation were observed.
Conclusions: Our analyses reveal that accumulation of p53 in endothelial cells contributes to blood vessel rarefaction and fibrosis during chronic cardiac pressure overload and suggest that endothelial cells may be a therapeutic target for preserving cardiac function during hypertrophy.
Keywords: angiogenesis; endothelium; fibrosis; heart failure; p53.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures





References
-
- Hein S, Arnon E, Kostin S, Schonburg M, Elsässer A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure‐overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003; 107:984-991. - PubMed
-
- Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy. Maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014; 114:1565-1571. - PubMed
-
- Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz‐Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J, Chien KR, Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci USA. 2001; 98:5780-5785. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

