Activation of PPARδ signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction
- PMID: 25713305
- PMCID: PMC4551122
- DOI: 10.1152/ajpheart.00679.2014
Activation of PPARδ signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction
Abstract
Exercise intolerance in heart failure has been linked to impaired skeletal muscle oxidative capacity. Oxidative metabolism and exercise capacity are regulated by PPARδ signaling. We hypothesized that PPARδ stimulation reverts skeletal muscle oxidative dysfunction. Myocardial infarction (MI) was induced in C57BL/6 mice and the development of ventricular dysfunction was monitored over 8 wk. Mice were randomized to the PPARδ agonist GW501516 (5 mg/kg body wt per day for 4 wk) or placebo 8 wk post-MI. Muscle function was assessed through running tests and grip strength measurements. In muscle, we analyzed muscle fiber cross-sectional area and fiber types, metabolic gene expression, fatty acid (FA) oxidation and ATP content. Signaling pathways were studied in C2C12 myotubes. FA oxidation and ATP levels decreased in muscle from MI mice compared with sham- operated mice. GW501516 administration increased oleic acid oxidation levels in skeletal muscle of the treated MI group compared with placebo treatment. This was accompanied by transcriptional changes including increased CPT1 expression. Further, the PPARδ-agonist improved running endurance compared with placebo. Cell culture experiments revealed protective effects of GW501516 against the cytokine-induced decrease of FA oxidation and changes in metabolic gene expression. Skeletal muscle dysfunction in HF is associated with impaired PPARδ signaling and treatment with the PPARδ agonist GW501516 corrects oxidative capacity and FA metabolism and improves exercise capacity in mice with LV dysfunction. Pharmacological activation of PPARδ signaling could be an attractive therapeutic intervention to counteract the progressive skeletal muscle dysfunction in HF.
Keywords: PPARδ; heart failure; metabolism; skeletal muscle.
Copyright © 2015 the American Physiological Society.
Figures

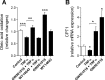
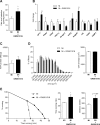
Comment in
-
Regulating PPARδ signaling as a potential therapeutic strategy for skeletal muscle disorders in heart failure.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H967-9. doi: 10.1152/ajpheart.00169.2015. Epub 2015 Mar 13. Am J Physiol Heart Circ Physiol. 2015. PMID: 25770240 Free PMC article. No abstract available.
References
-
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137: 354–366, 1996. - PubMed
-
- Coll T, Alvarez-Guardia D, Barroso E, Gomez-Foix AM, Palomer X, Laguna JC, Vazquez-Carrera M. Activation of peroxisome proliferator-activated receptor-delta by GW501516 prevents fatty acid-induced nuclear factor-kappaB activation and insulin resistance in skeletal muscle cells. Endocrinology 151: 1560–1569, 2010. - PubMed
-
- de Lange P, Ragni M, Silvestri E, Moreno M, Schiavo L, Lombardi A, Farina P, Feola A, Goglia F, Lanni A. Combined cDNA array/RT-PCR analysis of gene expression profile in rat gastrocnemius muscle: relation to its adaptive function in energy metabolism during fasting. FASEB J 18: 350–352, 2004. - PubMed
-
- Djouadi F, Aubey F, Schlemmer D, Bastin J. Peroxisome proliferator activated receptor delta (PPARdelta) agonist but not PPARalpha corrects carnitine palmitoyl transferase 2 deficiency in human muscle cells. J Clin Endocrinol Metab 90: 1791–1797, 2005. - PubMed
-
- Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

