Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production
- PMID: 25713338
- PMCID: PMC4378182
- DOI: 10.1104/pp.15.00107
Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boosts deoxynivalenol production
Abstract
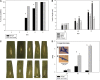
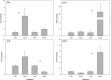
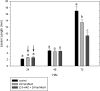
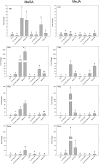
Priming refers to a mechanism whereby plants are sensitized to respond faster and/or more strongly to future pathogen attack. Here, we demonstrate that preexposure to the green leaf volatile Z-3-hexenyl acetate (Z-3-HAC) primed wheat (Triticum aestivum) for enhanced defense against subsequent infection with the hemibiotrophic fungus Fusarium graminearum. Bioassays showed that, after priming with Z-3-HAC, wheat ears accumulated up to 40% fewer necrotic spikelets. Furthermore, leaves of seedlings showed significantly smaller necrotic lesions compared with nonprimed plants, coinciding with strongly reduced fungal growth in planta. Additionally, we found that F. graminearum produced more deoxynivalenol, a mycotoxin, in the primed treatment. Expression analysis of salicylic acid (SA) and jasmonic acid (JA) biosynthesis genes and exogenous methyl salicylate and methyl jasmonate applications showed that plant defense against F. graminearum is sequentially regulated by SA and JA during the early and later stages of infection, respectively. Interestingly, analysis of the effect of Z-3-HAC pretreatment on SA- and JA-responsive gene expression in hormone-treated and pathogen-inoculated seedlings revealed that Z-3-HAC boosts JA-dependent defenses during the necrotrophic infection stage of F. graminearum but suppresses SA-regulated defense during its biotrophic phase. Together, these findings highlight the importance of temporally separated hormone changes in molding plant health and disease and support a scenario whereby the green leaf volatile Z-3-HAC protects wheat against Fusarium head blight by priming for enhanced JA-dependent defenses during the necrotrophic stages of infection.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures
References
-
- Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta 1734: 91–111 - PubMed
-
- Audenaert K, Van Broeck R, Bekaert B, De Witte F, Heremans B, Messens K, Hofte M, Haesaert G (2009) Fusarium head blight (FHB) in Flanders: population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur J Plant Pathol 125: 445–458
-
- Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16: 561–569 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

