Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function
- PMID: 25713362
- PMCID: PMC4364209
- DOI: 10.1073/pnas.1422373112
Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function
Erratum in
-
Correction.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):E1812. doi: 10.1073/pnas.1504383112. Epub 2015 Mar 11. Proc Natl Acad Sci U S A. 2015. PMID: 25762073 Free PMC article. No abstract available.
Abstract
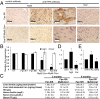
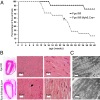
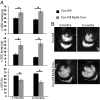
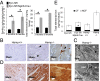
Iron is essential to the cell. Both iron deficiency and overload impinge negatively on cardiac health. Thus, effective iron homeostasis is important for cardiac function. Ferroportin (FPN), the only known mammalian iron-exporting protein, plays an essential role in iron homeostasis at the systemic level. It increases systemic iron availability by releasing iron from the cells of the duodenum, spleen, and liver, the sites of iron absorption, recycling, and storage respectively. However, FPN is also found in tissues with no known role in systemic iron handling, such as the heart, where its function remains unknown. To explore this function, we generated mice with a cardiomyocyte-specific deletion of Fpn. We show that these animals have severely impaired cardiac function, with a median survival of 22 wk, despite otherwise unaltered systemic iron status. We then compared their phenotype with that of ubiquitous hepcidin knockouts, a recognized model of the iron-loading disease hemochromatosis. The phenotype of the hepcidin knockouts was far milder, with normal survival up to 12 mo, despite far greater iron loading in the hearts. Histological examination demonstrated that, although cardiac iron accumulates within the cardiomyocytes of Fpn knockouts, it accumulates predominantly in other cell types in the hepcidin knockouts. We conclude, first, that cardiomyocyte FPN is essential for intracellular iron homeostasis and, second, that the site of deposition of iron within the heart determines the severity with which it affects cardiac function. Both findings have significant implications for the assessment and treatment of cardiac complications of iron dysregulation.
Keywords: cardiomyocyte; ferroportin; heart; hepcidin; iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Anker SD, et al. FAIR-HF Trial Investigators Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448. - PubMed
-
- Okonko DO, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: A randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51(2):103–112. - PubMed
-
- Klip IT, et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am Heart J. 2013;165(4):575–582, e3. - PubMed
-
- Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: Pathophysiology, diagnosis, and treatment. J Card Fail. 2010;16(11):888–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

