Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole
- PMID: 25713367
- PMCID: PMC4547261
- DOI: 10.1073/pnas.1421388112
Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole
Abstract
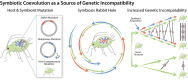
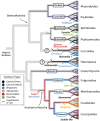
Many eukaryotes have obligate associations with microorganisms that are transmitted directly between generations. A model for heritable symbiosis is the association of aphids, a clade of sap-feeding insects, and Buchnera aphidicola, a gammaproteobacterium that colonized an aphid ancestor 150 million years ago and persists in almost all 5,000 aphid species. Symbiont acquisition enables evolutionary and ecological expansion; aphids are one of many insect groups that would not exist without heritable symbiosis. Receiving less attention are potential negative ramifications of symbiotic alliances. In the short run, symbionts impose metabolic costs. Over evolutionary time, hosts evolve dependence beyond the original benefits of the symbiosis. Symbiotic partners enter into an evolutionary spiral that leads to irreversible codependence and associated risks. Host adaptations to symbiosis (e.g., immune-system modification) may impose vulnerabilities. Symbiont genomes also continuously accumulate deleterious mutations, limiting their beneficial contributions and environmental tolerance. Finally, the fitness interests of obligate heritable symbionts are distinct from those of their hosts, leading to selfish tendencies. Thus, genes underlying the host-symbiont interface are predicted to follow a coevolutionary arms race, as observed for genes governing host-pathogen interactions. On the macroevolutionary scale, the rapid evolution of interacting symbiont and host genes is predicted to accelerate host speciation rates by generating genetic incompatibilities. However, degeneration of symbiont genomes may ultimately limit the ecological range of host species, potentially increasing extinction risk. Recent results for the aphid-Buchnera symbiosis and related systems illustrate that, whereas heritable symbiosis can expand ecological range and spur diversification, it also presents potential perils.
Keywords: Buchnera; Muller's ratchet; aphid; coevolution; selection levels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10(1):13–26. - PubMed
-
- Moran NA, Bennett GM. The tiniest tiny genomes. Annu Rev Microbiol. 2014;68:195–215. - PubMed
-
- Husnik F, et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell. 2013;153(7):1567–1578. - PubMed
-
- Nakabachi A, Ishida K, Hongoh Y, Ohkuma M, Miyagishima S-Y. Aphid gene of bacterial origin encodes a protein transported to an obligate endosymbiont. Curr Biol. 2014;24(14):R640–R641. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

