Differential fates of biomolecules delivered to target cells via extracellular vesicles
- PMID: 25713383
- PMCID: PMC4378439
- DOI: 10.1073/pnas.1418401112
Differential fates of biomolecules delivered to target cells via extracellular vesicles
Abstract
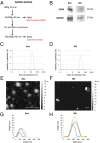
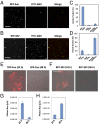
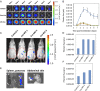
Extracellular vesicles (EVs), specifically exosomes and microvesicles (MVs), are presumed to play key roles in cell-cell communication via transfer of biomolecules between cells. The biogenesis of these two types of EVs differs as they originate from either the endosomal (exosomes) or plasma (MVs) membranes. To elucidate the primary means through which EVs mediate intercellular communication, we characterized their ability to encapsulate and deliver different types of macromolecules from transiently transfected cells. Both EV types encapsulated reporter proteins and mRNA but only MVs transferred the reporter function to recipient cells. De novo reporter protein expression in recipient cells resulted only from plasmid DNA (pDNA) after delivery via MVs. Reporter mRNA was delivered to recipient cells by both EV types, but was rapidly degraded without being translated. MVs also mediated delivery of functional pDNA encoding Cre recombinase in vivo to tissues in transgenic Cre-lox reporter mice. Within the parameters of this study, MVs delivered functional pDNA, but not RNA, whereas exosomes from the same source did not deliver functional nucleic acids. These results have significant implications for understanding the role of EVs in cellular communication and for development of EVs as delivery tools. Moreover, studies using EVs from transiently transfected cells may be confounded by a predominance of pDNA transfer.
Keywords: apoptotic body; cell communication; exosome; extracellular vesicle; microvesicle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Emerging picture of the distinct traits and functions of microvesicles and exosomes.Proc Natl Acad Sci U S A. 2015 Mar 24;112(12):3589-90. doi: 10.1073/pnas.1502590112. Epub 2015 Mar 11. Proc Natl Acad Sci U S A. 2015. PMID: 25762069 Free PMC article. No abstract available.
References
-
- EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. - PubMed
-
- Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1):R125–R134. - PubMed
-
- Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. - PubMed
-
- Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

