Specific disulfide cross-linking to constrict the mobile carrier domain of nonribosomal peptide synthetases
- PMID: 25713404
- PMCID: PMC4502403
- DOI: 10.1093/protein/gzv009
Specific disulfide cross-linking to constrict the mobile carrier domain of nonribosomal peptide synthetases
Abstract
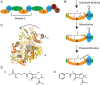
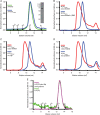
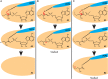
Nonribosomal peptide synthetases are large, multi-domain enzymes that produce peptide molecules with important biological activity such as antibiotic, antiviral, anti-tumor, siderophore and immunosuppressant action. The adenylation (A) domain catalyzes two reactions in the biosynthetic pathway. In the first reaction, it activates the substrate amino acid by adenylation and in the second reaction it transfers the amino acid onto the phosphopantetheine arm of the adjacent peptide carrier protein (PCP) domain. The conformation of the A domain differs significantly depending on which of these two reactions it is catalyzing. Recently, several structures of A-PCP di-domains have been solved using mechanism-based inhibitors to trap the PCP domain in the A domain active site. Here, we present an alternative strategy to stall the A-PCP di-domain, by engineering a disulfide bond between the native amino acid substrate and the A domain. Size exclusion studies showed a significant shift in apparent size when the mutant A-PCP was provided with cross-linking reagents, and this shift was reversible in the presence of high concentrations of reducing agent. The cross-linked protein crystallized readily in several of the conditions screened and the best crystals diffracted to ≈8 Å.
Keywords: crystallization; disulfide cross-link; nonribosomal peptide synthetase; protein engineering; thioesterification.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Challis G.L., Ravel J., Townsend C.A. (2000) Chem. Biol., 7, 211–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

