Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial
- PMID: 25713437
- PMCID: PMC5795709
- DOI: 10.1200/JCO.2014.56.6018
Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial
Abstract
Purpose: There is evidence from nonrandomized studies that a proportion of ipilimumab-treated patients with advanced melanoma experience long-term survival. To demonstrate a long-term survival benefit with ipilimumab, we evaluated the 5-year survival rates of patients treated in a randomized, controlled phase III trial.
Patients and methods: A milestone survival analysis was conducted to capture the 5-year survival rate of treatment-naive patients with advanced melanoma who received ipilimumab in a phase III trial. Patients were randomly assigned 1:1 to receive ipilimumab at 10 mg/kg plus dacarbazine (n = 250) or placebo plus dacarbazine (n = 252) at weeks 1, 4, 7, and 10 followed by dacarbazine alone every 3 weeks through week 22. Eligible patients could receive maintenance ipilimumab or placebo every 12 weeks beginning at week 24. A safety analysis was conducted on patients who survived at least 5 years and continued to receive ipilimumab as maintenance therapy.
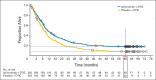
Results: The 5-year survival rate was 18.2% (95% CI, 13.6% to 23.4%) for patients treated with ipilimumab plus dacarbazine versus 8.8% (95% CI, 5.7% to 12.8%) for patients treated with placebo plus dacarbazine (P = .002). A plateau in the survival curve began at approximately 3 years. In patients who survived at least 5 years and continued to receive ipilimumab, grade 3 or 4 immune-related adverse events were observed exclusively in the skin.
Conclusion: The additional survival benefit of ipilimumab plus dacarbazine is maintained with twice as many patients alive at 5 years compared with those who initially received placebo plus dacarbazine. These results demonstrate a durable survival benefit with ipilimumab in advanced melanoma.
Trial registration: ClinicalTrials.gov NCT00324155.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- Robert C Thomas L Bondarenko I , etal: Ipilimumab plus dacarbazine for previously untreated metastatic melanoma N Engl J Med 364: 2517– 2526,2011. - PubMed
-
- Hoos A Ibrahim R Korman A , etal: Development of ipilimumab: Contribution to a new paradigm for cancer immunotherapy Semin Oncol 37: 533– 546,2010. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical