Regulation of intestinal immune system by dendritic cells
- PMID: 25713503
- PMCID: PMC4338263
- DOI: 10.4110/in.2015.15.1.1
Regulation of intestinal immune system by dendritic cells
Abstract
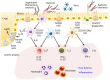
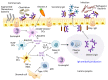
Innate immune cells survey antigenic materials beneath our body surfaces and provide a front-line response to internal and external danger signals. Dendritic cells (DCs), a subset of innate immune cells, are critical sentinels that perform multiple roles in immune responses, from acting as principal modulators to priming an adaptive immune response through antigen-specific signaling. In the gut, DCs meet exogenous, non-harmful food antigens as well as vast commensal microbes under steady-state conditions. In other instances, they must combat pathogenic microbes to prevent infections. In this review, we focus on the function of intestinal DCs in maintaining intestinal immune homeostasis. Specifically, we describe how intestinal DCs affect IgA production from B cells and influence the generation of unique subsets of T cell.
Keywords: Dendritic cells; Gut; Regulatory T cells; Secretory IgA; Th17.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

References
-
- Murphy K, Travers P, Walport M, Janeway C. Janeway's immunobiology. New York: Garland Science; pp. 466–468.
-
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. - PubMed
-
- Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol. 2011;187:733–747. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

