A wirelessly controlled implantable LED system for deep brain optogenetic stimulation
- PMID: 25713516
- PMCID: PMC4322607
- DOI: 10.3389/fnint.2015.00008
A wirelessly controlled implantable LED system for deep brain optogenetic stimulation
Abstract
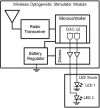
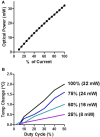
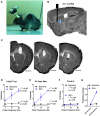
In recent years optogenetics has rapidly become an essential technique in neuroscience. Its temporal and spatial specificity, combined with efficacy in manipulating neuronal activity, are especially useful in studying the behavior of awake behaving animals. Conventional optogenetics, however, requires the use of lasers and optic fibers, which can place considerable restrictions on behavior. Here we combined a wirelessly controlled interface and small implantable light-emitting diode (LED) that allows flexible and precise placement of light source to illuminate any brain area. We tested this wireless LED system in vivo, in transgenic mice expressing channelrhodopsin-2 in striatonigral neurons expressing D1-like dopamine receptors. In all mice tested, we were able to elicit movements reliably. The frequency of twitches induced by high power stimulation is proportional to the frequency of stimulation. At lower power, contraversive turning was observed. Moreover, the implanted LED remains effective over 50 days after surgery, demonstrating the long-term stability of the light source. Our results show that the wireless LED system can be used to manipulate neural activity chronically in behaving mice without impeding natural movements.
Keywords: channelrhodopsin; direct pathway; freely-behaving; optogenetics; striatonigral; wireless.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

