A Cyclic Peptide Mimic of the β-Amyloid Binding Domain on Transthyretin
- PMID: 25713928
- PMCID: PMC4797071
- DOI: 10.1021/cn500272a
A Cyclic Peptide Mimic of the β-Amyloid Binding Domain on Transthyretin
Abstract
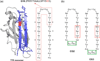
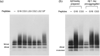
Self-association of β-amyloid (Aβ) into oligomers and fibrils is associated with Alzheimer's disease (AD), motivating the search for compounds that bind to and inhibit Aβ oligomerization and/or neurotoxicity. Peptides are an attractive class of such compounds, with potential advantages over small molecules in affinity and specificity. Self-complementation and peptide library screening are two strategies that have been employed in the search for peptides that bind to Aβ. Alternatively, one could design Aβ-binding peptides based on knowledge of complementary binding proteins. One candidate protein, transthyretin (TTR), binds Aβ, inhibits aggregation, and reduces its toxicity. Previously, strand G of TTR was identified as part of a specific Aβ binding domain, and G16, a 16-mer peptide with a sequence that spans strands G and H of TTR, was synthesized and tested. Although both TTR and G16 bound to Aβ, they differed significantly in their effect on Aβ aggregation, and G16 was less effective than TTR at protecting neurons from Aβ toxicity. G16 lacks the β-strand/loop/β-strand structure of TTR's Aβ binding domain. To enforce proper residue alignment, we transplanted the G16 sequence onto a β-hairpin template. Two peptides with 18 and 22 amino acids were synthesized using an orthogonally protected glutamic acid derivative, and an N-to-C cyclization reaction was carried out to further restrict conformational flexibility. The cyclized 22-mer (but not the noncyclized 22-mer nor the 18-mer) strongly suppressed Aβ aggregation into fibrils, and protected neurons against Aβ toxicity. The imposition of structural constraints generated a much-improved peptidomimetic of the Aβ binding epitope on TTR.
Keywords: Alzheimer’s disease; Beta-amyloid; amyloid fibrils; cyclic peptide; peptidomimetics; transthyretin.
Figures









References
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. - PubMed
-
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. - PubMed
-
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discovery. 2011;10:698–712. - PubMed
-
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81:136–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

