Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells
- PMID: 25714093
- PMCID: PMC4340623
- DOI: 10.1371/journal.pone.0117492
Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) promote adipocyte differentiation and inhibit inflammation in 3T3-F442A cells
Abstract
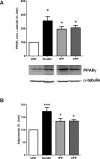
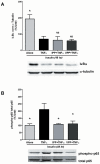
Milk derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) have shown promise as anti-hypertensive agents due to their inhibitory effects on angiotensin converting enzyme (ACE). Due to the key inter-related roles of hypertension, chronic inflammation and insulin resistance in the pathogenesis of metabolic syndrome, there is growing interest in investigating established anti-hypertensive agents for their effects on insulin sensitivity and inflammation. In this study, we examined the effects of IPP and VPP on 3T3-F442A murine pre-adipocytes, a widely used model for studying metabolic diseases. We found that both IPP and VPP induced beneficial adipogenic differentiation as manifested by intracellular lipid accumulation, upregulation of peroxisome proliferator-activated receptor gamma (PPARγ) and secretion of the protective lipid hormone adiponectin by these cells. The observed effects were similar to those induced by insulin, suggesting potential benefits in the presence of insulin resistance. IPP and VPP also inhibited cytokine induced pro-inflammatory changes such as reduction in adipokine levels and activation of the nuclear factor kappa B (NF-κB) pathway. Taken together, our findings suggest that IPP and VPP exert insulin-mimetic adipogenic effects and prevent inflammatory changes in adipocytes, which may offer protection against metabolic disease.
Conflict of interest statement
Figures



Similar articles
-
Milk-Derived Tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) Enhance Insulin Sensitivity and Prevent Insulin Resistance in 3T3-F442A Preadipocytes.J Agric Food Chem. 2018 Oct 3;66(39):10179-10187. doi: 10.1021/acs.jafc.8b02051. Epub 2018 Sep 20. J Agric Food Chem. 2018. PMID: 30160110
-
Milk-derived peptide Val-Pro-Pro (VPP) inhibits obesity-induced adipose inflammation via an angiotensin-converting enzyme (ACE) dependent cascade.Mol Nutr Food Res. 2015 Dec;59(12):2502-10. doi: 10.1002/mnfr.201500324. Epub 2015 Sep 29. Mol Nutr Food Res. 2015. PMID: 26346532
-
Beta-mecaptoethanol suppresses inflammation and induces adipogenic differentiation in 3T3-F442A murine preadipocytes.PLoS One. 2012;7(7):e40958. doi: 10.1371/journal.pone.0040958. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22911724 Free PMC article.
-
Gly-Ala-Gly-Val-Gly-Tyr, a novel synthetic peptide, improves glucose transport and exerts beneficial lipid metabolic effects in 3T3-L1 adipoctyes.Eur J Pharmacol. 2011 Jan 10;650(1):479-85. doi: 10.1016/j.ejphar.2010.10.006. Epub 2010 Oct 14. Eur J Pharmacol. 2011. PMID: 20951125
-
Casein hydrolysate containing the antihypertensive tripeptides Val-Pro-Pro and Ile-Pro-Pro improves vascular endothelial function independent of blood pressure-lowering effects: contribution of the inhibitory action of angiotensin-converting enzyme.Hypertens Res. 2007 Jun;30(6):489-96. doi: 10.1291/hypres.30.489. Hypertens Res. 2007. PMID: 17664851 Clinical Trial.
Cited by
-
A Pea (Pisum sativum L.) Seed Vicilins Hydrolysate Exhibits PPARγ Ligand Activity and Modulates Adipocyte Differentiation in a 3T3-L1 Cell Culture Model.Foods. 2020 Jun 16;9(6):793. doi: 10.3390/foods9060793. Foods. 2020. PMID: 32560200 Free PMC article.
-
Research on Bitter Peptides in the Field of Bioinformatics: A Comprehensive Review.Int J Mol Sci. 2024 Sep 12;25(18):9844. doi: 10.3390/ijms25189844. Int J Mol Sci. 2024. PMID: 39337334 Free PMC article. Review.
-
Whey Peptides Stimulate Differentiation and Lipid Metabolism in Adipocytes and Ameliorate Lipotoxicity-Induced Insulin Resistance in Muscle Cells.Nutrients. 2020 Feb 6;12(2):425. doi: 10.3390/nu12020425. Nutrients. 2020. PMID: 32041341 Free PMC article.
-
A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides.Food Chem X. 2022 Feb 2;13:100222. doi: 10.1016/j.fochx.2022.100222. eCollection 2022 Mar 30. Food Chem X. 2022. PMID: 35498998 Free PMC article.
-
Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments.Mar Drugs. 2022 Sep 30;20(10):626. doi: 10.3390/md20100626. Mar Drugs. 2022. PMID: 36286450 Free PMC article.
References
-
- Cleeman JI, Grundy SM, Becker D, Clark LT, Cooper RS, et al. (2001) Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Jama-Journal of the American Medical Association 285: 2486–2497. - PubMed
-
- Coppack S, Mohamed-Ali V, Karpe F (2005) Metabolic Syndrome: Insulin Resistance, Obesity, Diabetes Mellitus, Hypertension, Physical Activity and Genetic Factors. Cardiovascular Disease: Diet, Nutrition and Emerging Risk Factors: 22–49.
-
- Karagiannis A, Mikhailidis DP, Athyros VG, Kakafika AI, Tziomalos K, et al. (2007) The role of renin-angiotensin system inhibition in the treatment of hypertension in metabolic syndrome: are all the angiotensin receptor blockers equal? Expert Opinion on Therapeutic Targets 11: 191–205. - PubMed
-
- Sgarra L, Addabbo F, Potenza MA, Montagnani M (2012) Determinants of evolving metabolic and cardiovascular benefit/risk profiles of rosiglitazone therapy during the natural history of diabetes: molecular mechanisms in the context of integrated pathophysiology. American Journal of Physiology-Endocrinology and Metabolism 302: E1171–E1182. 10.1152/ajpendo.00038.2012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

