Disease-promoting effects of type I interferons in viral, bacterial, and coinfections
- PMID: 25714109
- PMCID: PMC4389918
- DOI: 10.1089/jir.2014.0227
Disease-promoting effects of type I interferons in viral, bacterial, and coinfections
Abstract
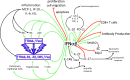
While type I interferons (IFNs) are universally acknowledged for their antiviral and immunostimulatory functions, there is increasing appreciation of the detrimental effects of inappropriate, excessive, or mistimed type I IFN responses in viral and bacterial infections. The underlying mechanisms by which type I IFNs promote susceptibility or severity include direct tissue damage by apoptosis induction or suppression of proliferation in tissue cells, immunopathology due to excessive inflammation, and cell death induced by TRAIL- and Fas-expressing immune cells, as well as immunosuppression through IL-10, IL-27, PD-L1, IL-1Ra, and other regulatory molecules that antagonize the induction or action of IL-1, IL-12, IL-17, IFN-γ, KC, and other effectors of the immune response. Bacterial superinfections following influenza infection are a prominent example of a situation where type I IFNs can misdirect the immune response. This review discusses current understanding of the parameters of signal strength, duration, timing, location, and cellular recipients that determine whether type I IFNs have beneficial or detrimental effects in infection.
Figures


References
-
- Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. 2010. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest 120(5):1674–1682 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
