RECIST 1.1 compared with RECIST 1.0 in patients with advanced renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy
- PMID: 25714313
- PMCID: PMC4352691
- DOI: 10.2214/AJR.14.13236
RECIST 1.1 compared with RECIST 1.0 in patients with advanced renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy
Abstract
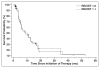
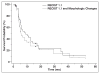
OBJECTIVE. Response Evaluation Criteria in Solid Tumors (RECIST) is the most widely accepted method to objectively assess response to therapy in renal cell carcinoma (RCC) treated with vascular endothelial growth factor (VEGF)-targeted therapy. Both RECIST 1.0 and 1.1 have been used to assess response to VEGF-targeted therapies; however, systematic comparisons are lacking. MATERIALS AND METHODS. Sixty-two patients with metastatic RCC treated with VEGF-targeted therapies were retrospectively studied. Tumor measurements and response assessment according to RECIST 1.1 and RECIST 1.0 were compared, including the number of target lesions, baseline measurements, response at each follow-up, best overall response, and time to progression (TTP). Morphologic changes and new enhancement were also assessed over the course of treatment, and TTP was evaluated using morphologic change criteria in combination with RECIST 1.1. RESULTS. The number of target lesions according to RECIST 1.1 was significantly fewer than by RECIST 1.0 (median, 2 vs 4; p < 0.0001). At first imaging follow-up, the percentage change of the sums of the diameter measurements by RECIST 1.1 and RECIST 1.0 were highly concordant (R = 0.857; mean shrinkage, 12.1% by RECIST 1.1 vs 10.8% by RECIST 1.0). Best response assessment was highly concordant between the two criteria (weighted κ = 0.819). There was no evidence of a difference in TTP by the two criteria, with a median TTP of 8.9 months (95% CI for the median, 5.5-13.9) by RECIST 1.1 and 8.9 months (95% CI for the median, 5.8-13.6) by RECIST 1.0. The median TTP by RECIST 1.1 alone was 8.9 months compared with 5.6 months for RECIST 1.1 and morphologic changes combined. CONCLUSION. RECIST 1.1 and RECIST 1.0 response assessments were overall highly concordant in patients with RCC treated with VEGF-targeted therapy, with fewer target lesions according to RECIST 1.1 but no difference in TTP.
Keywords: CT; Response Evaluation Criteria in Solid Tumors (RECIST); renal cell carcinoma; tumor response assessment; vascular endothelial growth factor–targeted therapy.
Figures
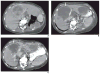
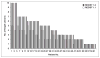
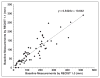
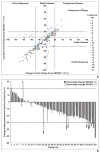
References
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–247. - PubMed
-
- Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1. 1: what oncologists want to know and what radiologists need to know. AJR. 2010;195:281–289. - PubMed
-
- Piatek CI, Desai BB, Wei-Tsao D, et al. J Clin Oncol. suppl. Vol. 29. American Society of Clinical Oncology; 2011. [Accessed November 17, 2014]. RECIST 1.0 versus 1.1: implications for trial interpretation and design in advanced prostate cancer; p. abstr 2563. website meetinglibrary.asco.org/content/84825-102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

