The cellobiose sensor CebR is the gatekeeper of Streptomyces scabies pathogenicity
- PMID: 25714708
- PMCID: PMC4358012
- DOI: 10.1128/mBio.02018-14
The cellobiose sensor CebR is the gatekeeper of Streptomyces scabies pathogenicity
Abstract
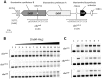
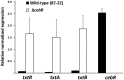
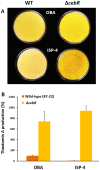
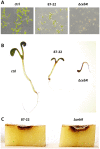
A relatively small number of species in the large genus Streptomyces are pathogenic; the best characterized of these is Streptomyces scabies. The pathogenicity of S. scabies strains is dependent on the production of the nitrated diketopiperazine thaxtomin A, which is a potent plant cellulose synthesis inhibitor. Much is known about the genetic loci associated with plant virulence; however, the molecular mechanisms by which S. scabies triggers expression of thaxtomin biosynthetic genes, beyond the pathway-specific activator TxtR, are not well understood. In this study, we demonstrate that binding sites for the cellulose utilization repressor CebR occur and function within the thaxtomin biosynthetic cluster. This was an unexpected result, as CebR is devoted to primary metabolism and nutritive functions in nonpathogenic streptomycetes. In S. scabies, cellobiose and cellotriose inhibit the DNA-binding ability of CebR, leading to an increased expression of the thaxtomin biosynthetic and regulatory genes txtA, txtB, and txtR. Deletion of cebR results in constitutive thaxtomin A production and hypervirulence of S. scabies. The pathogenicity of S. scabies is thus under dual direct positive and negative transcriptional control where CebR is the cellobiose-sensing key that locks the expression of txtR, the key necessary to unlock the production of the phytotoxin. Interestingly, CebR-binding sites also lie upstream of and within the thaxtomin biosynthetic clusters in Streptomyces turgidiscabies and Streptomyces acidiscabies, suggesting that CebR is most likely an important regulator of virulence in these plant-pathogenic species as well.
Importance: What makes a microorganism pathogenic is not limited to the genes acquired for virulence. Using the main causative agent of scab lesions on root and tuber crops as an example, our work identified the subtle but essential genetic changes that generate the cis-acting elements necessary for proper timing of the expression of the cluster of genes responsible for the biosynthesis of thaxtomin A, the primary virulence factor in plant-pathogenic streptomycetes. These data illustrate a situation in which a regulator associated with primary metabolism in nonpathogens, CebR, has been coopted as a master regulator of virulence in pathogenic species. Furthermore, the manipulation of CebR-mediated control of thaxtomin production will facilitate overproduction of this natural and biodegradable herbicide for commercial purposes. Our work thus provides a concrete example of how a strictly theoretical and computational work was able to elucidate a regulatory mechanism associated with the virulence of a plant pathogen and to generate solutions to purely agro-industrial concerns.
Copyright © 2015 Francis et al.
Figures
References
-
- Hopwood DA. 2007. Streptomyces in nature and medicine: the antibiotic makers. Oxford University Press, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

