Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes
- PMID: 25714716
- PMCID: PMC4358007
- DOI: 10.1128/mBio.02554-14
Chronic superantigen exposure induces systemic inflammation, elevated bloodstream endotoxin, and abnormal glucose tolerance in rabbits: possible role in diabetes
Abstract
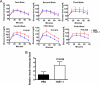
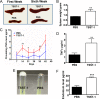
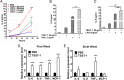
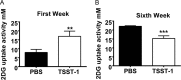
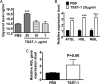
Excessive weight and obesity are associated with the development of diabetes mellitus type 2 (DMII) in humans. They also pose high risks of Staphylococcus aureus colonization and overt infections. S. aureus causes a wide range of severe illnesses in both healthy and immunocompromised individuals. Among S. aureus virulence factors, superantigens are essential for pathogenicity. In this study, we show that rabbits that are chronically exposed to S. aureus superantigen toxic shock syndrome toxin-1 (TSST-1) experience impaired glucose tolerance, systemic inflammation, and elevated endotoxin levels in the bloodstream, all of which are common findings in DMII. Additionally, such DMII-associated findings are also seen through effects of TSST-1 on isolated adipocytes. Collectively, our findings suggest that chronic exposure to S. aureus superantigens facilitates the development of DMII, which may lead to therapeutic targeting of S. aureus and its superantigens.
Importance: Obesity has a strong correlation with type 2 diabetes, in which fatty tissue, containing adipocytes, contributes to the development of the illness through altered metabolism and chronic inflammation. The human microbiome changes in persons with obesity and type 2 diabetes, including increases in Staphylococcus aureus colonization and overt infections. While the microbiome is essential for human wellness, there is little understanding of the role of microbes in obesity or the development of diabetes. Here, we demonstrate that the S. aureus superantigen toxic shock syndrome toxin-1 (TSST-1), an essential exotoxin in pathogenesis, induces inflammation, lipolysis, and insulin resistance in adipocytes both in vitro and in vivo. Chronic stimulation of rabbits with TSST-1 results in impaired systemic glucose tolerance, the hallmark finding in type 2 diabetes in humans, suggesting a role of S. aureus and its superantigens in the progression to type 2 diabetes.
Copyright © 2015 Vu et al.
Figures


Similar articles
-
Contribution of toxic shock syndrome toxin-1 to systemic inflammation investigated by a mouse model of cervicovaginal infection with Staphylococcus aureus.Med Microbiol Immunol. 2018 Nov;207(5-6):297-306. doi: 10.1007/s00430-018-0551-4. Epub 2018 Jul 6. Med Microbiol Immunol. 2018. PMID: 29980843
-
Effects of a new extracorporeal system using CTR on mortality and inflammatory responses to bacterial toxin-induced multiple organ dysfunction syndrome in rabbits.Blood Purif. 2006;24(3):327-34. doi: 10.1159/000091997. Epub 2006 Mar 13. Blood Purif. 2006. PMID: 16534195
-
Glucose Mediates Niche-Specific Repression of Staphylococcus aureus Toxic Shock Syndrome Toxin-1 through the Activity of CcpA in the Vaginal Environment.J Bacteriol. 2022 Oct 18;204(10):e0026922. doi: 10.1128/jb.00269-22. Epub 2022 Sep 15. J Bacteriol. 2022. PMID: 36106854 Free PMC article.
-
Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses.Crit Rev Microbiol. 1990;17(4):251-72. doi: 10.3109/10408419009105728. Crit Rev Microbiol. 1990. PMID: 2206394 Review.
-
Toxic-shock syndrome: a commentary and review of the characteristics of Staphylococcus aureus strains.Infection. 1983 Jul-Aug;11(4):181-8. doi: 10.1007/BF01641192. Infection. 1983. PMID: 6352506 Review.
Cited by
-
Nutritional Effects of the Enteral Nutritional Formula on Regulation of Gut Microbiota and Metabolic Level in Type 2 Diabetes Mellitus Mice.Diabetes Metab Syndr Obes. 2021 Apr 28;14:1855-1869. doi: 10.2147/DMSO.S301454. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 33953585 Free PMC article.
-
Exposome-wide Association Study for Metabolic Syndrome.Front Genet. 2021 Dec 7;12:783930. doi: 10.3389/fgene.2021.783930. eCollection 2021. Front Genet. 2021. PMID: 34950191 Free PMC article. No abstract available.
-
Kawasaki syndrome: role of superantigens revisited.FEBS J. 2021 Mar;288(6):1771-1777. doi: 10.1111/febs.15512. Epub 2020 Aug 24. FEBS J. 2021. PMID: 32770775 Free PMC article. Review.
-
The role of pathogens in diabetes pathogenesis and the potential of immunoproteomics as a diagnostic and prognostic tool.Front Microbiol. 2022 Nov 14;13:1042362. doi: 10.3389/fmicb.2022.1042362. eCollection 2022. Front Microbiol. 2022. PMID: 36483212 Free PMC article. Review.
-
Host Cationic Antimicrobial Molecules Inhibit S. aureus Exotoxin Production.mSphere. 2023 Feb 21;8(1):e0057622. doi: 10.1128/msphere.00576-22. Epub 2023 Jan 4. mSphere. 2023. PMID: 36598227 Free PMC article.
References
-
- International Diabetes Federation 2013. Global burden of diabetes. Diabetic atlas, 6th ed Brussels, Belgium: Accessed 17 October 2014 http://www.idf.org/diabetesatlas.
-
- Creely SJ, McTernan PG, Kusminski CM, Fisher FM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. 2007. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292:E740–E747. doi:10.1152/ajpendo.00302.2006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
