The protein kinase promiscuities in the cancer-preventive mechanisms of NSAIDs
- PMID: 25714784
- PMCID: PMC4885542
- DOI: 10.1097/CEJ.0000000000000129
The protein kinase promiscuities in the cancer-preventive mechanisms of NSAIDs
Abstract
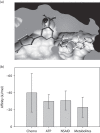
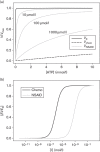
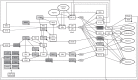
NSAIDs have been observed to have cancer-preventive properties, but the actual mechanism is elusive. We hypothesize that NSAIDs might have an effect through common pathways and targets of anticancer drugs by exploiting promiscuities of anticancer drug targets. Here, we have explored NSAIDs by their structural and pharmacophoric similarities with small anticancer molecules. In-silico analyses have shown a strong similarity between NSAIDs and protein kinase (PK) inhibitors. The calculated affinities of NSAIDs were found to be lower than the affinities of anticancer drugs, but higher than the affinities of compounds that are not specific to PKs. The competitive inhibition model suggests that PK might be inhibited by around 10%, which was confirmed by biochemical screening of some NSAIDs against PKs. NSAIDs did not affect all PKs universally, but had specificities for certain sets of PKs, which differed according to the NSAID. The study revealed potentially new features and mechanisms of NSAIDs that are useful in explaining their role in cancer prevention, which might lead to clinically significant breakthroughs in the future.
Figures



References
-
- An X, Tiwari AK, Sun Y, Ding PR, Ashby CR, Jr, Chen ZS. (2010). BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res 34:1255–1268. - PubMed
-
- Arora A, Scholar E. (2005). Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315:971–979. - PubMed
-
- Balkwill F, Mantovani A. (2001). Inflammation and cancer: back to Virchow? Lancet 357:539–545. - PubMed
-
- Baselga J. (2006). Targeting tyrosine kinases in cancer: the second wave. Science 312:1175–1178. - PubMed
-
- Bathaie SZ, Nikfarjam L, Rahmanpour R, Moosavi-Movahedi AA. (2010). Spectroscopic studies of the interaction of aspirin and its important metabolite, salicylate ion, with DNA, A·T and G·C rich sequences. Spectrochim Acta A Mol Biomol Spectrosc 77:1077–1083. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

