Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA
- PMID: 25714897
- PMCID: PMC4500635
- DOI: 10.1021/acschembio.5b00005
Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA
Abstract
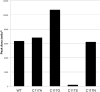
Nonreducing polyketide synthases (NR-PKSs) are unique among PKSs in their domain structure, notably including a starter unit:acyl-carrier protein (ACP) transacylase (SAT) domain that selects an acyl group as the primer for biosynthesis, most commonly acetyl-CoA from central metabolism. This clan of mega-enzymes resembles fatty acid synthases (FASs) by sharing both their central chain elongation steps and their capacity for iterative catalysis. In this mode of synthesis, catalytic domains involved in chain extension exhibit substrate plasticity to accommodate growing chains as small as two carbons to 20 or more. PksA is the NR-PKS central to the biosynthesis of the mycotoxin aflatoxin B1 whose SAT domain accepts an unusual hexanoyl starter from a dedicated yeast-like FAS. Explored in this paper is the ability of PksA to utilize a selection of potential starter units as substrates to initiate and sustain extension and cyclization to on-target, programmed polyketide synthesis. Most of these starter units were successfully accepted and properly processed by PksA to achieve biosynthesis of the predicted naphthopyrone product. Analysis of the on-target and derailment products revealed trends of tolerance by individual PksA domains to alternative starter units. In addition, natural and un-natural variants of the active site cysteine were examined and found to be capable of biosynthesis, suggesting possible direct loading of starter units onto the β-ketoacyl synthase (KS) domain. In light of the data assembled here, the predictable synthesis of unnatural products by NR-PKSs is more fully defined.
Figures



Similar articles
-
Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase.Proc Natl Acad Sci U S A. 2006 Nov 7;103(45):16728-33. doi: 10.1073/pnas.0604112103. Epub 2006 Oct 27. Proc Natl Acad Sci U S A. 2006. PMID: 17071746 Free PMC article.
-
Interrogation of global active site occupancy of a fungal iterative polyketide synthase reveals strategies for maintaining biosynthetic fidelity.J Am Chem Soc. 2012 Apr 18;134(15):6865-77. doi: 10.1021/ja3016389. Epub 2012 Apr 9. J Am Chem Soc. 2012. PMID: 22452347 Free PMC article.
-
Deconstruction of iterative multidomain polyketide synthase function.Science. 2008 Apr 11;320(5873):243-6. doi: 10.1126/science.1154711. Science. 2008. PMID: 18403714 Free PMC article.
-
Fungal type III polyketide synthases.Nat Prod Rep. 2014 Oct;31(10):1306-17. doi: 10.1039/c4np00096j. Nat Prod Rep. 2014. PMID: 25182423 Review.
-
The Structural Enzymology of Iterative Aromatic Polyketide Synthases: A Critical Comparison with Fatty Acid Synthases.Annu Rev Biochem. 2018 Jun 20;87:503-531. doi: 10.1146/annurev-biochem-063011-164509. Annu Rev Biochem. 2018. PMID: 29925265 Review.
Cited by
-
Tautomycetin Synthetic Analogues: Selective Inhibitors of Protein Phosphatase I.ChemMedChem. 2021 Mar 3;16(5):839-850. doi: 10.1002/cmdc.202000801. Epub 2020 Dec 10. ChemMedChem. 2021. PMID: 33301228 Free PMC article.
-
Intrinsic and Extrinsic Programming of Product Chain Length and Release Mode in Fungal Collaborating Iterative Polyketide Synthases.J Am Chem Soc. 2020 Oct 7;142(40):17093-17104. doi: 10.1021/jacs.0c07050. Epub 2020 Sep 23. J Am Chem Soc. 2020. PMID: 32833442 Free PMC article.
-
The architectures of iterative type I PKS and FAS.Nat Prod Rep. 2018 Oct 17;35(10):1046-1069. doi: 10.1039/c8np00039e. Nat Prod Rep. 2018. PMID: 30137093 Free PMC article. Review.
-
Diversity-Oriented Combinatorial Biosynthesis of Hybrid Polyketide Scaffolds from Azaphilone and Benzenediol Lactone Biosynthons.Org Lett. 2016 Mar 18;18(6):1262-5. doi: 10.1021/acs.orglett.6b00110. Epub 2016 Mar 2. Org Lett. 2016. PMID: 26934205 Free PMC article.
-
Insights into 6-Methylsalicylic Acid Bio-assembly by Using Chemical Probes.Angew Chem Int Ed Engl. 2016 Mar 1;55(10):3463-7. doi: 10.1002/anie.201509038. Epub 2016 Feb 2. Angew Chem Int Ed Engl. 2016. PMID: 26833898 Free PMC article.
References
-
- Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A. Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat Prod Rep. 2007;24:162–190. - PubMed
-
- Kirschning A, Hahn F. Merging chemical synthesis and biosynthesis: a new chapter in the total synthesis of natural products and natural product libraries. Angew Chem Int Ed Engl. 2012;51:4012–4022. - PubMed
-
- Moore BS, Hertweck C. Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Natural Product Reports. 2002;19:70–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

