Intranasal vaccination with a plant-derived H5 HA vaccine protects mice and ferrets against highly pathogenic avian influenza virus challenge
- PMID: 25714901
- PMCID: PMC4514375
- DOI: 10.4161/21645515.2014.988554
Intranasal vaccination with a plant-derived H5 HA vaccine protects mice and ferrets against highly pathogenic avian influenza virus challenge
Abstract
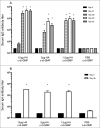
Highly pathogenic avian influenza H5N1 infection remains a public health threat and vaccination is the best measure of limiting the impact of a potential pandemic. Mucosal vaccines have the advantage of eliciting immune responses at the site of viral entry, thereby preventing infection as well as further viral transmission. In this study, we assessed the protective efficacy of hemagglutinin (HA) from the A/Indonesia/05/05 (H5N1) strain of influenza virus that was produced by transient expression in plants. The plant-derived vaccine, in combination with the mucosal adjuvant (3',5')-cyclic dimeric guanylic acid (c-di-GMP) was used for intranasal immunization of mice and ferrets, before challenge with a lethal dose of the A/Indonesia/05/05 (H5N1) virus. Mice vaccinated with 15 μg or 5 μg of adjuvanted HA survived the viral challenge, while all control mice died within 10 d of challenge. Vaccinated animals elicited serum hemagglutination inhibition, IgG and IgA antibody titers. In the ferret challenge study, all animals vaccinated with the adjuvanted plant vaccine survived the lethal viral challenge, while 50% of the control animals died. In both the mouse and ferret models, the vaccinated animals were better protected from weight loss and body temperature changes associated with H5N1 infection compared with the non-vaccinated controls. Furthermore, the systemic spread of the virus was lower in the vaccinated animals compared with the controls. Results presented here suggest that the plant-produced HA-based influenza vaccine adjuvanted with c-di-GMP is a promising vaccine/adjuvant combination for the development of new mucosal influenza vaccines.
Keywords: (3′; 5′)-cyclic dimeric guanylic acid; adjuvant; c-di-GMP; ferret infection model; influenza H5N1; intranasal vaccination; mice, plant vaccine.
Figures


Similar articles
-
Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice.Vaccine. 2011 Jul 12;29(31):4973-82. doi: 10.1016/j.vaccine.2011.04.094. Epub 2011 May 18. Vaccine. 2011. PMID: 21600260
-
Flagellin-HA vaccines protect ferrets and mice against H5N1 highly pathogenic avian influenza virus (HPAIV) infections.Vaccine. 2012 Nov 6;30(48):6833-8. doi: 10.1016/j.vaccine.2012.09.013. Epub 2012 Sep 18. Vaccine. 2012. PMID: 23000130
-
M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza.Vaccine. 2017 Jul 24;35(33):4177-4183. doi: 10.1016/j.vaccine.2017.06.039. Epub 2017 Jun 28. Vaccine. 2017. PMID: 28668565 Free PMC article.
-
New strategies for the development of H5N1 subtype influenza vaccines: progress and challenges.BioDrugs. 2011 Oct 1;25(5):285-98. doi: 10.1007/BF03256169. BioDrugs. 2011. PMID: 21942913 Review.
-
The current state of H5N1 vaccines and the use of the ferret model for influenza therapeutic and prophylactic development.J Infect Dev Ctries. 2012 Jun 15;6(6):465-9. doi: 10.3855/jidc.2666. J Infect Dev Ctries. 2012. PMID: 22706187 Review.
Cited by
-
The Last Ten Years of Advancements in Plant-Derived Recombinant Vaccines against Hepatitis B.Int J Mol Sci. 2016 Oct 13;17(10):1715. doi: 10.3390/ijms17101715. Int J Mol Sci. 2016. PMID: 27754367 Free PMC article. Review.
-
Production of complex viral glycoproteins in plants as vaccine immunogens.Plant Biotechnol J. 2018 Jun 11;16(9):1531-45. doi: 10.1111/pbi.12963. Online ahead of print. Plant Biotechnol J. 2018. PMID: 29890031 Free PMC article. Review.
-
Mucosal and Systemic Immune Responses to Influenza H7N9 Antigen HA1-2 Co-Delivered Intranasally with Flagellin or Polyethyleneimine in Mice and Chickens.Front Immunol. 2017 Apr 5;8:326. doi: 10.3389/fimmu.2017.00326. eCollection 2017. Front Immunol. 2017. PMID: 28424686 Free PMC article.
-
FcRn-Targeted Mucosal Vaccination against Influenza Virus Infection.J Immunol. 2021 Sep 1;207(5):1310-1321. doi: 10.4049/jimmunol.2100297. Epub 2021 Aug 11. J Immunol. 2021. PMID: 34380652 Free PMC article.
-
High accumulation in tobacco seeds of hemagglutinin antigen from avian (H5N1) influenza.Transgenic Res. 2017 Dec;26(6):775-789. doi: 10.1007/s11248-017-0047-9. Epub 2017 Oct 6. Transgenic Res. 2017. PMID: 28986672
References
-
- World Health Organization Summary of assessment as of January 2015. Available from: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IR....
-
- Zhang Y, Zhang Q, Kong H, Jiang Y, Gao Y, Deng G, Shi J, Tian G, Liu L, Liu J, et al. . H5N1 hybrid viruses bearing 2009/H1N1 virus genes transmit in guinea pigs by respiratory droplet. Science 2013; 340:1459-63; PMID:23641061; http://dx.doi.org/10.1126/science.1229455 - DOI - PubMed
-
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. . Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534-41; PMID:22723413; http://dx.doi.org/10.1126/science.1213362 - DOI - PMC - PubMed
-
- Chichester JA, Haaheim LR, Yusibov V. Using plant cells as influenza vaccine substrates. Expert Rev Vaccin 2009; 8:493-8; PMID:19348564; http://dx.doi.org/10.1586/erv.09.3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
