Role of radiology in central nervous system stimulation
- PMID: 25715044
- PMCID: PMC4651263
- DOI: 10.1259/bjr.20140507
Role of radiology in central nervous system stimulation
Abstract
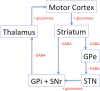
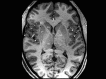
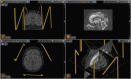
Central nervous system (CNS) stimulation is becoming increasingly prevalent. Deep brain stimulation (DBS) has been proven to be an invaluable treatment for movement disorders and is also useful in many other neurological conditions refractory to medical treatment, such as chronic pain and epilepsy. Neuroimaging plays an important role in operative planning, target localization and post-operative follow-up. The use of imaging in determining the underlying mechanisms of DBS is increasing, and the dependence on imaging is likely to expand as deep brain targeting becomes more refined. This article will address the expanding role of radiology and highlight issues, including MRI safety concerns, that radiologists may encounter when confronted with a patient with CNS stimulation equipment in situ.
Figures




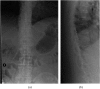
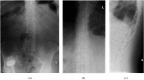
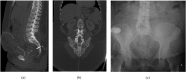
References
-
- Standring S. Gray's anatomy: the anatomical basis of clinical practice. Edinburgh, UK: Churchill Livingstone Elsevier; 2008.
-
- Starr PA, Barbaro NM, Larson PS. Neurosurgical operative atlas: functional neurosurgery. 2nd edn. Stuttgart, Germany: Thieme; 2009.
-
- Pereira EA, Green AL, Nandi D, Aziz TZ. Deep brain stimulation: indications and evidence. Expert Rev Med Devices 2007; 4: 591–603. - PubMed
-
- Vilensky JA, Gilman S. Horsley was the first to use electrical stimulation of the human cerebral cortex intraoperatively. Surg Neurol 2002; 58: 425–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

