Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex
- PMID: 25715284
- PMCID: PMC4612443
- DOI: 10.1093/cercor/bhv019
Complementary Patterns of Direct Amygdala and Hippocampal Projections to the Macaque Prefrontal Cortex
Abstract
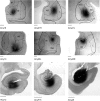
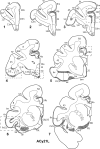
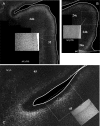
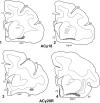
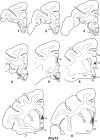
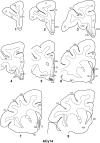
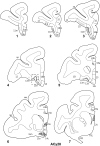
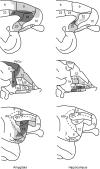
The projections from the amygdala and hippocampus (including subiculum and presubiculum) to prefrontal cortex were compared using anterograde tracers injected into macaque monkeys (Macaca fascicularis, Macaca mulatta). Almost all prefrontal areas were found to receive some amygdala inputs. These connections, which predominantly arose from the intermediate and magnocellular basal nucleus, were particularly dense in parts of the medial and orbital prefrontal cortex. Contralateral inputs were not, however, observed. The hippocampal projections to prefrontal areas were far more restricted, being confined to the ipsilateral medial and orbital prefrontal cortex (within areas 11, 13, 14, 24a, 32, and 25). These hippocampal projections principally arose from the subiculum, with the fornix providing the sole route. Thus, while the lateral prefrontal cortex essentially receives only amygdala inputs, the orbital prefrontal cortex receives both amygdala and hippocampal inputs, though these typically target different areas. Only in medial prefrontal cortex do direct inputs from both structures terminate in common sites. But, even when convergence occurs within an area, the projections predominantly terminate in different lamina (hippocampal inputs to layer III and amygdala inputs to layers I, II, and VI). The resulting segregation of prefrontal inputs could enable the parallel processing of different information types in prefrontal cortex.
Keywords: anatomy; emotion; fornix; hippocampus; memory; subiculum.
© The Author 2015. Published by Oxford University Press.
Figures






References
-
- Admon R, Milad MR, Hendler T. 2013. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sc. 17:337–347. - PubMed
-
- Aggleton JP. 1986. A description of the amygdalo-hippocampal interconnections in the macaque monkey. Exp Brain Res. 64:515–526. - PubMed
-
- Aggleton JP. 1985. A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res. 57:390–399. - PubMed
-
- Aggleton JP. 2012. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 36:1579–1596. - PubMed
-
- Aggleton JP, Burton MJ, Passingham RE. 1980. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 190:347–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

