Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn's disease
- PMID: 25715355
- PMCID: PMC4819603
- DOI: 10.1136/gutjnl-2014-306919
Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn's disease
Abstract
Background and aim: Thymus-derived regulatory T cells (Tregs) mediate dominant peripheral tolerance and treat experimental colitis. Tregs can be expanded from patient blood and were safely used in recent phase 1 studies in graft versus host disease and type 1 diabetes. Treg cell therapy is also conceptually attractive for Crohn's disease (CD). However, barriers exist to this approach. The stability of Tregs expanded from Crohn's blood is unknown. The potential for adoptively transferred Tregs to express interleukin-17 and exacerbate Crohn's lesions is of concern. Mucosal T cells are resistant to Treg-mediated suppression in active CD. The capacity for expanded Tregs to home to gut and lymphoid tissue is unknown.
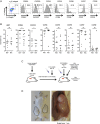
Methods: To define the optimum population for Treg cell therapy in CD, CD4(+)CD25(+)CD127(lo)CD45RA(+) and CD4(+)CD25(+)CD127(lo)CD45RA(-) Treg subsets were isolated from patients' blood and expanded in vitro using a workflow that can be readily transferred to a good manufacturing practice background.
Results: Tregs can be expanded from the blood of patients with CD to potential target dose within 22-24 days. Expanded CD45RA(+) Tregs have an epigenetically stable FOXP3 locus and do not convert to a Th17 phenotype in vitro, in contrast to CD45RA(-) Tregs. CD45RA(+) Tregs highly express α4β7 integrin, CD62L and CC motif receptor 7 (CCR7). CD45RA(+) Tregs also home to human small bowel in a C.B-17 severe combined immune deficiency (SCID) xenotransplant model. Importantly, in vitro expansion enhances the suppressive ability of CD45RA(+) Tregs. These cells also suppress activation of lamina propria and mesenteric lymph node lymphocytes isolated from inflamed Crohn's mucosa.
Conclusions: CD4(+)CD25(+)CD127(lo)CD45RA(+) Tregs may be the most appropriate population from which to expand Tregs for autologous Treg therapy for CD, paving the way for future clinical trials.
Keywords: IBD; IBD BASIC RESEARCH; IBD CLINICAL; IMMUNOTHERAPY; INTESTINAL T CELLS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures




References
-
- Morrissey PJ, Charrier K, Braddy S, et al. . CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med 1993;178:237–44. 10.1084/jem.178.1.237 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT088747MA/WT_/Wellcome Trust/United Kingdom
- RG/13/12/30395/BHF_/British Heart Foundation/United Kingdom
- G0802068/MRC_/Medical Research Council/United Kingdom
- MR/J006742/1/MRC_/Medical Research Council/United Kingdom
- MR/L022699/1/MRC_/Medical Research Council/United Kingdom
- MR/K025538/1/MRC_/Medical Research Council/United Kingdom
- MR/M003493/1/MRC_/Medical Research Council/United Kingdom
- G0801537/ID/MRC_/Medical Research Council/United Kingdom
- MR/K002996/1/MRC_/Medical Research Council/United Kingdom
- G0800746/MRC_/Medical Research Council/United Kingdom
- 091009/WT_/Wellcome Trust/United Kingdom
- 88245/MRC_/Medical Research Council/United Kingdom
- MR/N006445/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G0801537/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
