A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation
- PMID: 25715393
- PMCID: PMC4352987
- DOI: 10.1242/dev.105536
A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation
Abstract
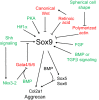
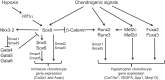
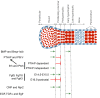
Decades of work have identified the signaling pathways that regulate the differentiation of chondrocytes during bone formation, from their initial induction from mesenchymal progenitor cells to their terminal maturation into hypertrophic chondrocytes. Here, we review how multiple signaling molecules, mechanical signals and morphological cell features are integrated to activate a set of key transcription factors that determine and regulate the genetic program that induces chondrogenesis and chondrocyte differentiation. Moreover, we describe recent findings regarding the roles of several signaling pathways in modulating the proliferation and maturation of chondrocytes in the growth plate, which is the 'engine' of bone elongation.
Keywords: Chondrocyte hypertrophy; Chondrogenesis; Fgfr3; Growth plate; Ihh; PTHrP; Sox9.
© 2015. Published by The Company of Biologists Ltd.
Figures



References
-
- Akiyama H., Chaboissier M.-C., Martin J. F., Schedl A. and De Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828 10.1101/gad.1017802 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

