Dasatinib in high-risk core binding factor acute myeloid leukemia in first complete remission: a French Acute Myeloid Leukemia Intergroup trial
- PMID: 25715404
- PMCID: PMC4450623
- DOI: 10.3324/haematol.2014.114884
Dasatinib in high-risk core binding factor acute myeloid leukemia in first complete remission: a French Acute Myeloid Leukemia Intergroup trial
Abstract
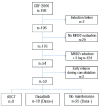
Core-binding factor acute myeloid leukemia is a favorable acute myeloid leukemia subset cytogenetically defined by t(8;21) or inv(16)/t(16;16) rearrangements, disrupting RUNX1 (previously CBFA/AML1) or CBFB transcription factor functions. The receptor tyrosine kinase KIT is expressed in the vast majority of these acute myeloid leukemias and frequent activating KIT gene mutations have been associated with a higher risk of relapse. This phase II study aimed to evaluate dasatinib as maintenance therapy in patients with core-binding factor acute myeloid leukemia in first hematologic complete remission, but at higher risk of relapse due to molecular disease persistence or recurrence. A total of 26 patients aged 18-60 years old previously included in the CBF-2006 trial were eligible to receive dasatinib 140 mg daily if they had a poor initial molecular response (n=18) or a molecular recurrence (n=8). The tolerance of dasatinib as maintenance therapy was satisfactory. The 2-year disease-free survival in this high-risk population of patients was 25.7%. All but one patient with molecular recurrence presented subsequent hematologic relapse. Patients with slow initial molecular response had a similar disease-free survival when treated with dasatinib (40.2% at 2 years) or without any maintenance (50.0% at 2 years). The disappearance of KIT gene mutations at relapse suggests that clonal devolution may in part explain the absence of efficacy observed with single-agent dasatinib in these patients (n. EudraCT: 2006-006555-12).
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. - PubMed
-
- Wang Q, Stacy T, Miller JD, et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell. 1996; 87(4):697–708. - PubMed
-
- Dombret H, Preudhomme C, Boissel N. Core binding factor acute myeloid leukemia (CBF-AML): is high-dose Ara-C (HDAC) consolidation as effective as you think? Curr Opin Hematol. 2009; 16(2):92–97. - PubMed
-
- Yin JAL, O’Brien MA, Hills RK, et al. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012; 120(14):2826–2835. - PubMed
-
- Boissel N, Leroy H, Brethon B, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia. 2006;20(6):965–970. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

