Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems
- PMID: 25715710
- PMCID: PMC4341198
- DOI: 10.1038/srep08587
Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems
Abstract
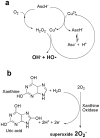
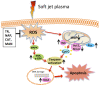
In this study, we assessed the role of different reactive oxygen species (ROS) generated by soft jet plasma and chemical-induced ROS systems with regard to cell death in T98G, A549, HEK293 and MRC5 cell lines. For a comparison with plasma, we generated superoxide anion (O2(-)), hydroxyl radical (HO·), and hydrogen peroxide (H2O2) with chemicals inside an in vitro cell culture. Our data revealed that plasma decreased the viability and intracellular ATP values of cells and increased the apoptotic population via a caspase activation mechanism. Plasma altered the mitochondrial membrane potential and eventually up-regulated the mRNA expression levels of BAX, BAK1 and H2AX gene but simultaneously down-regulated the levels of Bcl-2 in solid tumor cells. Moreover, a western blot analysis confirmed that plasma also altered phosphorylated ERK1/2/MAPK protein levels. At the same time, using ROS scavengers with plasma, we observed that scavengers of HO· (mannitol) and H2O2 (catalase and sodium pyruvate) attenuated the activity of plasma on cells to a large extent. In contrast, radicals generated by specific chemical systems enhanced cell death drastically in cancer as well as normal cell lines in a dose-dependent fashion but not specific with regard to the cell type as compared to plasma.
Figures






References
-
- Devasagayam T. P. et al. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India 52, 794–804 (2004). - PubMed
-
- Simon H. U., Haj-Yehia A. & Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5, 415–8 (2000). - PubMed
-
- Trachootham D., Alexandre J. & Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 (2009). - PubMed
-
- Dobbelstein M. & Moll U. Targeting tumour-supportive cellular machineries in anticancer drug development. Nat. Rev. Drug Discov. 13, 179–196 (2014). - PubMed
-
- Zeng J. Y. et al. Synergistic activities of MET/RON inhibitor BMS-777607 and mTOR inhibitor AZD8055 to polyploid cells derived from pancreatic cancer and cancer stem cells. Mol. Cancer Ther. 13, 37–48 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

