Complement C1q-induced activation of β-catenin signalling causes hypertensive arterial remodelling
- PMID: 25716000
- PMCID: PMC4351572
- DOI: 10.1038/ncomms7241
Complement C1q-induced activation of β-catenin signalling causes hypertensive arterial remodelling
Abstract
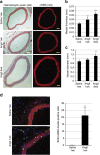
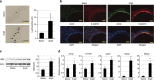
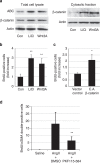
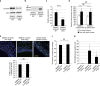
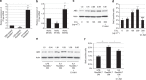
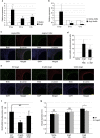
Hypertension induces structural remodelling of arteries, which leads to arteriosclerosis and end-organ damage. Hyperplasia of vascular smooth muscle cells (VSMCs) and infiltration of immune cells are the hallmark of hypertensive arterial remodelling. However, the precise molecular mechanisms of arterial remodelling remain elusive. We have recently reported that complement C1q activates β-catenin signalling independent of Wnts. Here, we show a critical role of complement C1-induced activation of β-catenin signalling in hypertensive arterial remodelling. Activation of β-catenin and proliferation of VSMCs were observed after blood-pressure elevation, which were prevented by genetic and chemical inhibition of β-catenin signalling. Macrophage depletion and C1qa gene deletion attenuated the hypertension-induced β-catenin signalling, proliferation of VSMCs and pathological arterial remodelling. Our findings unveil the link between complement C1 and arterial remodelling and suggest that C1-induced activation of β-catenin signalling becomes a novel therapeutic target to prevent arteriosclerosis in patients with hypertension.
Figures



Comment in
-
Hypertension: Complement C1 and β-catenin in hypertensive arterial remodelling.Nat Rev Nephrol. 2015 May;11(5):255. doi: 10.1038/nrneph.2015.31. Epub 2015 Mar 17. Nat Rev Nephrol. 2015. PMID: 25776562 No abstract available.
References
-
- Horton R. GBD 2010: understanding disease, injury, and risk. Lancet 380, 2053–2054 (2012). - PubMed
-
- Kearney P. M. et al.. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005). - PubMed
-
- Gibbons G. H. & Dzau V. J. The emerging concept of vascular remodeling. N. Engl. J. Med. 330, 1431–1438 (1994). - PubMed
-
- Intengan H. D. & Schiffrin E. L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38, 581–587 (2001). - PubMed
-
- Li C. & Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 12, 435–445 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

