Cell dynamics and gene expression control in tissue homeostasis and development
- PMID: 25716053
- PMCID: PMC4358661
- DOI: 10.15252/msb.20145549
Cell dynamics and gene expression control in tissue homeostasis and development
Abstract
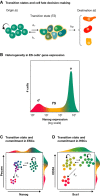
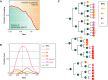
During tissue and organ development and maintenance, the dynamic regulation of cellular proliferation and differentiation allows cells to build highly elaborate structures. The development of the vertebrate retina or the maintenance of adult intestinal crypts, for instance, involves the arrangement of newly created cells with different phenotypes, the proportions of which need to be tightly controlled. While some of the basic principles underlying these processes developing and maintaining these organs are known, much remains to be learnt from how cells encode the necessary information and use it to attain those complex but reproducible arrangements. Here, we review the current knowledge on the principles underlying cell population dynamics during tissue development and homeostasis. In particular, we discuss how stochastic fate assignment, cell division, feedback control and cellular transition states interact during organ and tissue development and maintenance in multicellular organisms. We propose a framework, involving the existence of a transition state in which cells are more susceptible to signals that can affect their gene expression state and influence their cell fate decisions. This framework, which also applies to systems much more amenable to quantitative analysis like differentiating embryonic stem cells, links gene expression programmes with cell population dynamics.
Keywords: development; differentiation; homeostasis; stochastic cell fate; transition state.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

