Enantioselective cross-coupling of meso-epoxides with aryl halides
- PMID: 25716775
- PMCID: PMC4415026
- DOI: 10.1021/jacs.5b01909
Enantioselective cross-coupling of meso-epoxides with aryl halides
Abstract
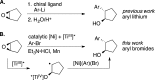
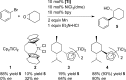
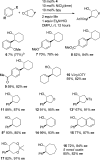
The first enantioselective cross-electrophile coupling of aryl bromides with meso-epoxides to form trans-β-arylcycloalkanols is presented. The reaction is catalyzed by a combination of (bpy)NiCl2 and a chiral titanocene under reducing conditions. Yields range from 57 to 99% with 78-95% enantiomeric excess. The 30 examples include a variety of functional groups (ether, ester, ketone, nitrile, ketal, trifluoromethyl, sulfonamide, sulfonate ester), both aryl and vinyl halides, and five- to seven-membered rings. The intermediacy of a carbon radical is strongly suggested by the conversion of cyclooctene monoxide to an aryl [3.3.0]bicyclooctanol.
Figures



References
-
- Nielsen L. P. C.; Jacobsen E. N.. Catalytic Asymmetric Epoxide Ring-Opening Chemistry. In Aziridines and Epoxides in Organic Synthesis; Yudin A. K., Ed.; Wiley-VCH: Weinheim, Germany, 2006; Chapter 7, pp 229–269.
- Huang C.-Y.; Doyle A. G. Chem. Rev. 2014, 114, 8153–8198. - PubMed
-
- Jacobsen E. N. Acc. Chem. Res. 2000, 33, 421–431. - PubMed
- Martínez L. E.; Leighton J. L.; Carsten D. H.; Jacobsen E. N. J. Am. Chem. Soc. 1995, 117, 5897–5898.
- Jacobsen E. N.; Kakiuchi F.; Konsler R. G.; Larrow J. F.; Tokunaga M. Tetrahedron Lett. 1997, 38, 773–776.
- Tao B.; Lo M. M.-C.; Fu G. C. J. Am. Chem. Soc. 2001, 123, 353–354. - PubMed
- Bartoli G.; Bosco M.; Carlone A.; Locatelli M.; Massaccesi M.; Melchiorre P.; Sambri L. Org. Lett. 2004, 6, 2173–2176. - PubMed
- Kalow J. A.; Doyle A. G. J. Am. Chem. Soc. 2010, 132, 3268–3269. - PubMed
- Nielsen L. P. C.; Zuend S. J.; Ford D. D.; Jacobsen E. N. J. Org. Chem. 2012, 77, 2486–2495. - PMC - PubMed
- Ingle G.; Mormino M. G.; Antilla J. C. Org. Lett. 2014, 16, 5548–5551. - PMC - PubMed
- Liu Y.; Ren W.-M.; Liu J.; Lu X.-B. Angew. Chem., Int. Ed. 2013, 52, 11594–11598. - PubMed
- Ellis W. C.; Jung Y.; Mulzer M.; Di Girolamo R.; Lobkovsky E. B.; Coates G. W. Chem. Sci. 2014, 5, 4004–4011.
-
- Cole B. M.; Shimizu K. D.; Krueger C. A.; Harrity J. P. A.; Snapper M. L.; Hoveyda A. H. Angew. Chem., Int. Ed. Engl. 1996, 35, 1668–1671.
- Shimizu K. D.; Cole B. M.; Krueger C. A.; Kuntz K. W.; Snapper M. L.; Hoveyda A. H. Angew. Chem., Int. Ed. Engl. 1997, 36, 1704–1707.
- Schaus S. E.; Jacobsen E. N. Org. Lett. 2000, 2, 1001–1004. - PubMed
- Palkulski Z.; Pietrusiewicz K. M. Tetrahedron: Asymmetry 2004, 15, 41–45.
-
-
For a review of transition-metal-catalyzed cross-coupling with epoxides, see ref (1b). Although no enantioselective examples have been reported, several enantiospecific reactions are known.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

