A placebo-controlled trial of dextromethorphan as an adjunct in opioid-dependent patients undergoing methadone maintenance treatment
- PMID: 25716777
- PMCID: PMC4540107
- DOI: 10.1093/ijnp/pyv008
A placebo-controlled trial of dextromethorphan as an adjunct in opioid-dependent patients undergoing methadone maintenance treatment
Abstract
Background: Low-dose dextromethorphan (DM) might have anti-inflammatory and neurotrophic effects mechanistically remote from an NMDA receptor. In a randomized, double-blind, controlled 12 week study, we investigated whether add-on dextromethorphan reduced cytokine levels and benefitted opioid-dependent patients undergoing methadone maintenance therapy (MMT).
Methods: Patients were randomly assigned to a group: DM60 (60mg/day dextromethorphan; n = 65), DM120 (120mg/day dextromethorphan; n = 65), or placebo (n = 66). Primary outcomes were the methadone dose required, plasma morphine level, and retention in treatment. Plasma tumor necrosis factor (TNF)-α, C-reactive protein, interleukin (IL)-6, IL-8, transforming growth factor-β1, and brain-derived neurotrophic factor (BDNF) levels were examined during weeks 0, 1, 4, 8, and 12. Multiple linear regressions with generalized estimating equation methods were used to examine the therapeutic effect.
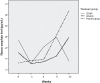
Results: After 12 weeks, the DM60 group had significantly longer treatment retention and lower plasma morphine levels than did the placebo group. Plasma TNF-α was significantly decreased in the DM60 group compared to the placebo group. However, changes in plasma cytokine levels, BDNF levels, and the methadone dose required in the three groups were not significantly different.
Conclusions: We provide evidence-decreased concomitant heroin use-of low-dose add-on DM's efficacy for treating opioid-dependent patients undergoing MMT.
Keywords: Cytokines; Dextromethorphan; Heroin; cytokine; dextromethorphan; methadone maintenance therapy; opioid dependence.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures

References
-
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P. (2007). Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J Psychopharmacol 21:820–825. - PubMed
-
- Bisaga A, Popik P. (2000). In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend 59:1–15. - PubMed
-
- Bisaga A, Gianelli P, Popik P. (1997). Opiate withdrawal with dextromethorphan. Am J Psych 154:584. - PubMed
-
- Bolanos CA, Nestler EJ. (2004). Neurotrophic mechanisms in drug addiction. Neuromol Med 5:69–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

